Here, we present a simplified sample extraction protocol, followed by a single LC-MS/MS injection to quantify both Budesonide and Formoterol in the same run, achieving LLOQ’s of 5 pg/mL for both analytes.
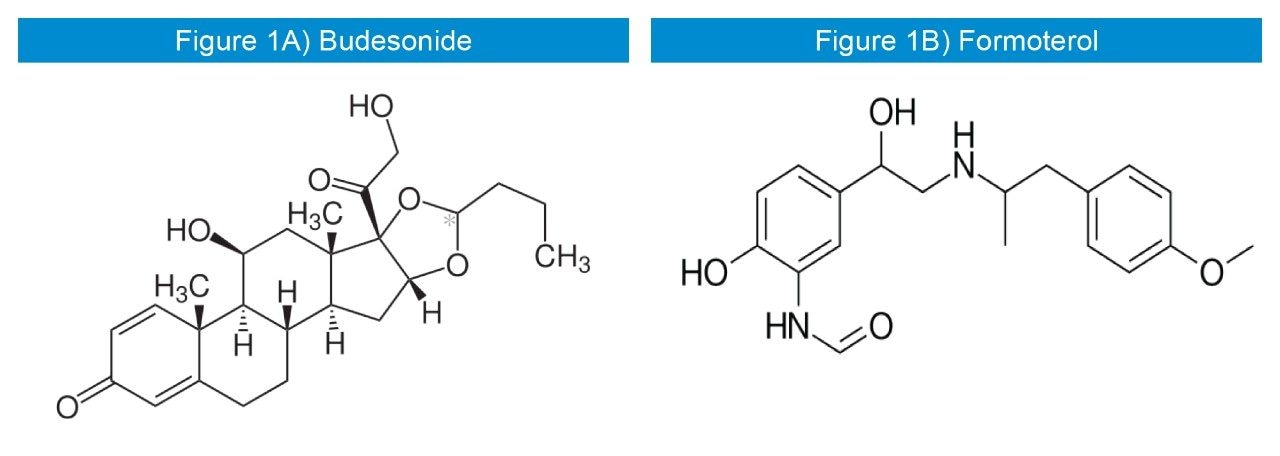
Budesonide (Figure 1A) is a widely used corticosteroid sold under the brand name Pulmicort, among others.1 It is delivered via an inhaler, pill, nasal spray, and rectal forms.1 The inhaled form is used in the long-term management of asthma and chronic obstructive pulmonary disease (COPD).1
Formoterol (Figure 1B) is a long-acting β2 agonist (LABA) used as a bronchodilator in the management of asthma and COPD. Formoterol has an extended duration of action (up to 12 h) compared to short-acting β2 agonists such as salbutamol (albuterol), which are effective for 4 h to 6 h. LABAs such as Formoterol are used as “symptom controllers” to supplement prophylactic corticosteroid therapy. A “reliever” short-acting β2 agonist (e.g., salbutamol) is still required, since LABAs are not recommended for the treatment of acute asthma. It was patented in 1972 and came into medical use in 1998.1 It is also marketed in the combination formulations such as Budesonide/Formoterol. Inhalation of the two pharmaceutical ingredients as one dose in combination inhalers has proved to be more clinically effective,2 and therefore a method for the concurrent bioanalysis of Budesonide and Formoterol is required.
Since these molecules are delivered as a nasal formulation, their levels in circulating fluids are very low. As a result, extremely sensitive analytical methods are required for pharmacokinetics, bioanalysis, or bioequivalence. Distinct physico-chemical properties of Budesonide and Formoterol, specifically their logP (2.42 and 1.91) and pKa (13.74 and 8.61) values make their analysis within the same run challenging. Most analytical methods described in literature use either two separate extractions and LC-MS/MS conditions, or the same extraction protocol and two different LC-MS/MS conditions.3 As a result, the sample analysis time and cost are significantly increased. Here, we present a simplified sample extraction protocol, followed by a single LC-MS/MS injection to quantify both Budesonide and Formoterol in the same run, achieving LLOQ’s of 5 pg/mL for both analytes.
Budesonide and Formoterol were solubilized in DMSO and then diluted down in 70:30 waters:acetonitrile. The working stock solution was then used to spike human plasma (BioIVT, MA, USA) to make a calibration curve from 5–1000 pg/mL. QC samples were then spiked at 10, 100, and 750 pg/mL. 500 μL of each of the calibration curve and QC samples (in triplicate) were pre-treated with 500 μL of 4% phosphoric acid in water mixed. These pre-treated samples were then extracted using Oasis WCX 96-well μElution plates using the protocol below.
|
Prime: |
200 μL methanol |
|
Equilibrate: |
200 μL water |
|
Load sample: |
Pre-treated sample was loaded onto the extraction plate in two steps of ~500 μL each |
|
Wash: |
200 μL of 5% methanol in water |
|
Elute: |
2 × 25 μL 50:50 isopropanol/methanol (v/v) |
|
Dilute: |
50 μL water |
|
LC conditions |
|
|---|---|
|
LC system: |
ACQUITY UPLC I-Class PLUS |
|
Detection: |
Xevo TQ-XS Mass Spectrometer, ESI+ |
|
Column: |
ACQUITY UPLC BEH C18, 130 Å, 1.7 μm, 2.1 mm × 50 mm |
|
Temp.: |
55 °C |
|
Sample temp.: |
5 °C |
|
Injection volume: |
10 μL |
|
Mobile phase A: |
0.1% ammonium hydroxide in water |
|
Mobile phase B: |
0.1% ammonium hydroxide in acetonitrile |
|
LC-MS software: |
MassLynx (v4.2) |
|
Quantification software: |
TargetLynx |
|
Capillary: |
3 kV |
|
Cone voltage: |
80 V |
|
Desolvation temp.: |
400 °C |
|
Cone gas flow: |
150 L/Hr |
|
Desolvation gas flow: |
800 L/Hr |
|
Collision gas flow: |
0.15 mL/min |
|
Nebulizer gas flow: |
7 Bar |
All steps in sample preparation, LC, and MS method were optimized during method development to ensure that analytes of interest are adequately separated from other matrix components and maximum sensitivity is achieved.
Budesonide and Formoterol were spiked in human plasma to generate a calibration curve from 5–1000 pg/mL and QC’s at 10 pg/mL (LQC), 100 pg/mL (MQC) and 750 pg/mL (HQC). These spiked plasma samples were used for optimization of sample extraction. A modified version of the Oasis 2x4 method development approach was used for initial sorbent and pre-treatment screening. Based on the pKa of the analytes, Oasis WCX, and Oasis MCX SPE sorbents were evaluated. For each sorbent type, acidic and basic pre-treatment prior to SPE were also evaluated. Budesonide showed comparable recoveries across the different sorbents and different pre-treatment conditions. Formoterol however, showed much higher recoveries on the WCX sorbent when coupled with acid pre-treatment (data not shown). The final protocol used for sample extraction is described in the experimental section above.
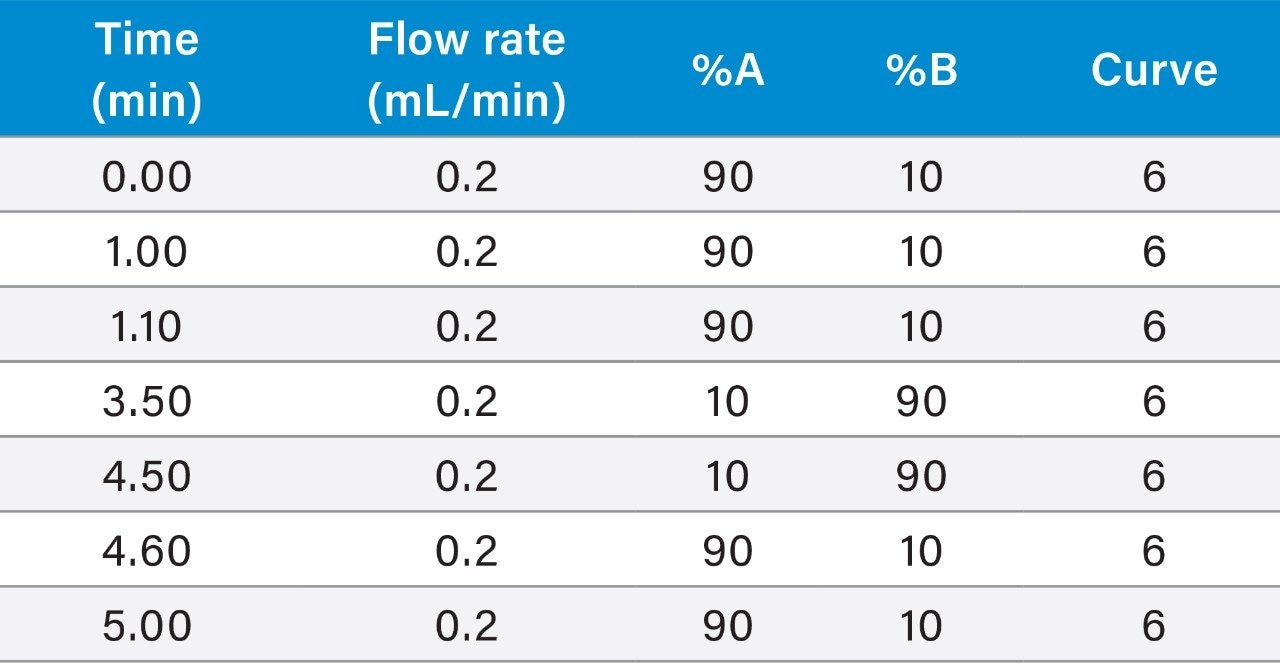
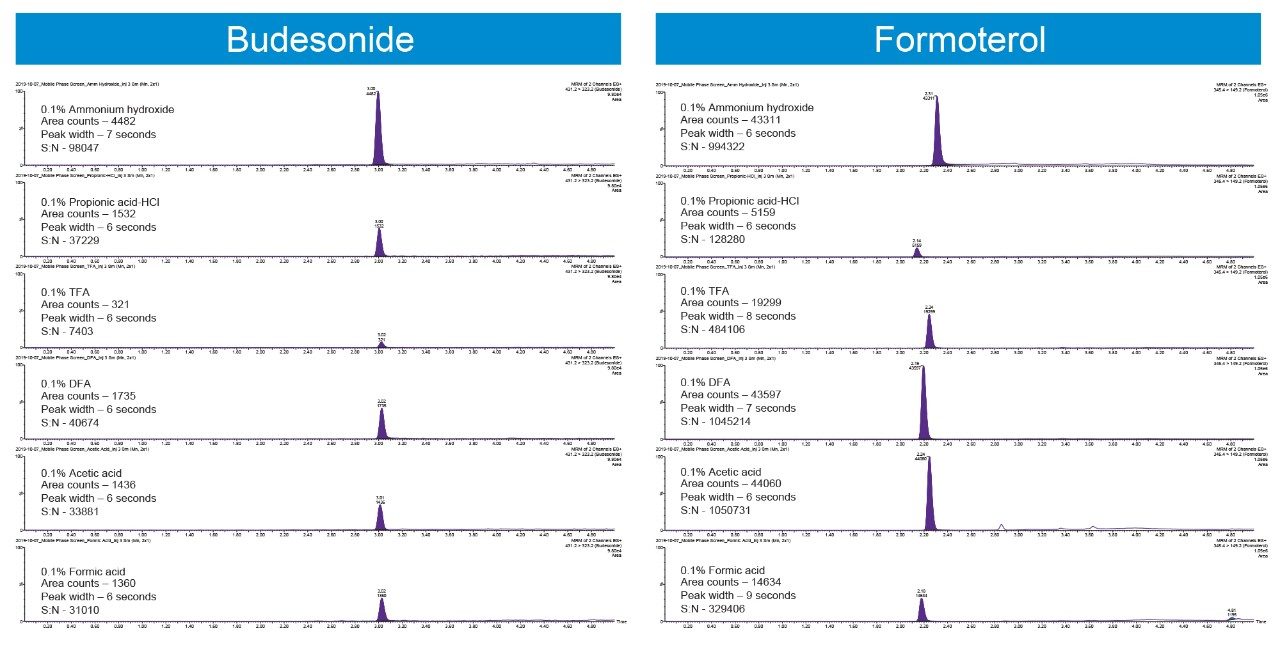
The physico-chemical properties of Budesonide and Formoterol make them ideally suited for a reversed-phase chromatographic separation. Multiple reversed-phase columns, including BEH C18, HSS T3, HSS C18, and BEH Phenyl were evaluated and BEH C18 gave the best chromatographic performance for both analytes (data not shown). Additionally, flow rate, mobile phase additives, and gradient conditions can also have a significant impact on peak shapes and signal to noise. After evaluating flow rates from 200–500 μL/min and different gradient starting conditions, flow rate of 200 μL/min and initial gradient conditions of 90:10 mobile phase A:B were employed. Ammonium hydroxide, triflouroacetic acid, diflouroacetic acid, propionic acid, acetic acid, and formic acid were evaluated as possible mobile phase additives (Figure 2). 0.1 % acetic acid gave the highest response based on peak area and best signal to noise for Formoterol, whereas 0.1% ammonium hydroxide achieved the best chromatographic performance for Budesonide.
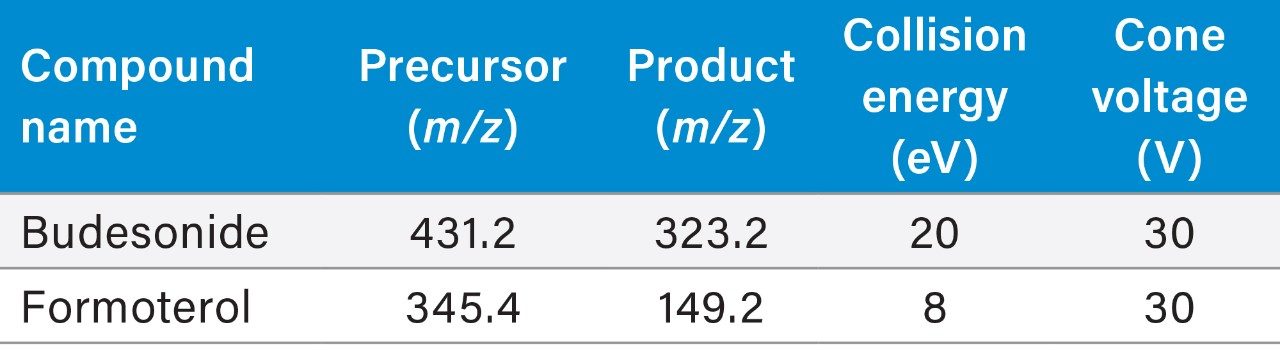
The Xevo TQ-XS tandem quadrupole mass spectrometer operating in positive ion electrospray mode was used to quantify Budesonide and Formoterol. MRM transitions listed in the methods section were used for quantification. Source conditions and tune page parameters which impact the analyte responses were optimized. Capillary voltage had a measurable impact on the sensitivities for both analytes (Figure 3).
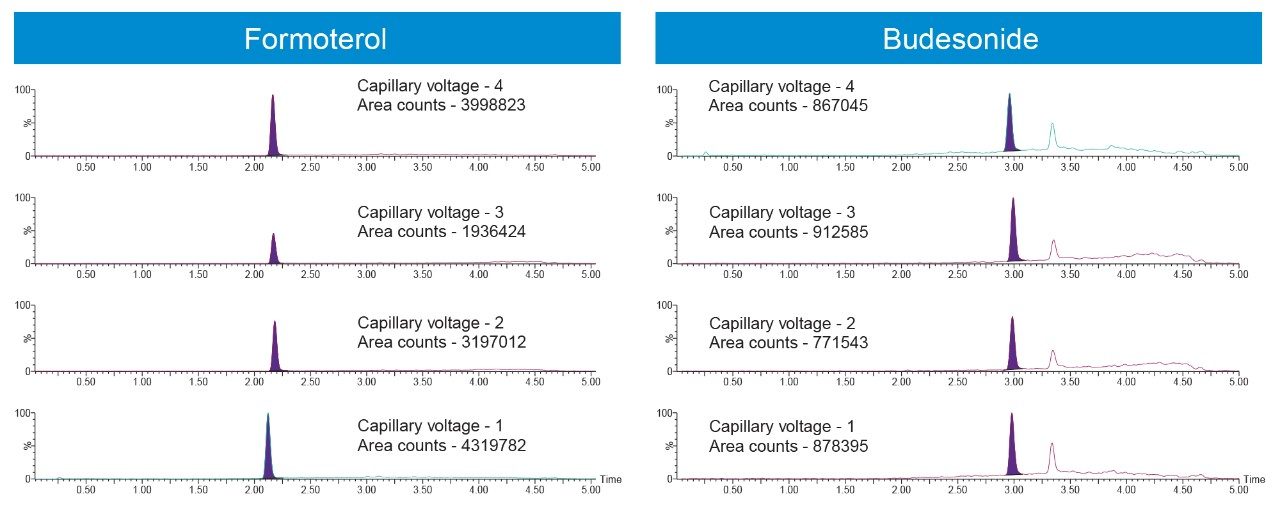
Because the aim of the method was to quantify Formoterol and Budesonide in the same injection, and because ionization efficiency for Budesonide is lower than that of Formoterol under the given conditions, every attempt was made to increase the sensitivity for Budesonide. Therefore, even though capillary voltage of 1 kV gave much higher area counts for Formoterol, capillary voltage of 3 kV was used in the final method to increase the sensitivity for Budesonide. Based on the data, the sensitivity for Formoterol could be increased by almost 2-fold by using a capillary voltage of 1 kV if needed. Similarly, 0.1% ammonium hydroxide was used as the mobile phase additive as it gave much higher area counts and S:N for Budesonide. If an assay is needed to quantify Formoterol alone, using 0.1% acetic acid can significantly improve the sensitivity, and therefore allow for a much lower LLOQ.
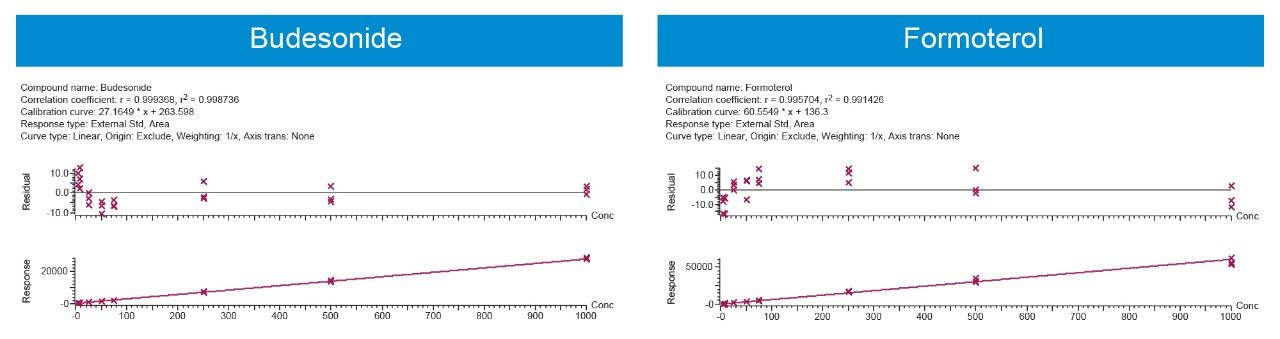
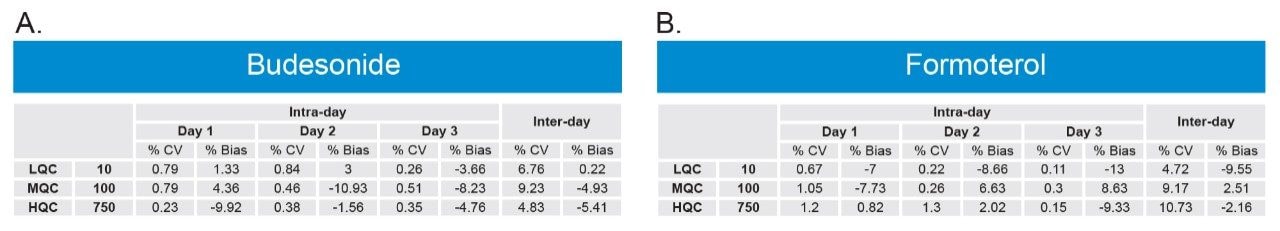
Using 500 μL of serum and the sample preparation strategy described previously, quantification limits of 5 pg/mL for both analytes were achieved based on %CV <20%. Calibration curves (5–1000 pg/mL) were linear with R2 values >0.99 (1/x weighted regression) (Figure 4). Intra and inter-day precision and accuracy (3 replicates per day across 3 days) for both analytes was excellent with mean % RSDs all <11%. QC performance is highlighted in Tables 1A (Budesonide) and 1B (Formoterol). This method provides a balance between the quantification for both analytes in a single injection. Since this method uses a single extraction and LC-MS/MS injection, it can save cost and instrument time compared to previously described methodologies.3 The work presented here also provides a framework to further optimize and fine-tune the method if quantification of only one of the analytes is required.
The method described employs a simple pretreatment and SPE sample preparation strategy combined with analytical flow LC and tandemquadrupole MS for pg/mL level quantification of Budesonide and Formoterol from human plasma. The main features of the method include:
720006767, February 2020