A Smart Clean-up Approach to Reduce Isobaric Interference for Multi-Residue Pesticide Analysis, Based Upon LC-MS/MS, of the Difficult Matrices, Black Tea, and Cocoa Beans
Dies ist ein Applikationsbericht, der keinen detaillierten Abschnitt zu Versuchen enthält.
Abstract
Applying a generic QuEChERS protocol without clean-up is challenging when faced with complex food commodities due to the abundance of endogenous components, such as fats, phospholipids, pigments, and other phytochemicals. These components are known to cause matrix effects and isobaric interference, which negatively impact detection and quantitation of pesticide residues. Adding a clean-up step after extraction helps to remove matrix components to obtain more reliable results, improving sensitivity and selectivity, and maintaining instrument robustness. Using Solid-Phase Extraction (SPE) with a pass-through protocol allows pesticides to pass through the stationary phase, whereas the matrix components are retained on the SPE sorbent material.
The objective of this work was to establish the performance of a method based upon QuEChERS but using a simple pass-through SPE cleanup with an Oasis™ PRiME HLB cartridge prior to UPLC-MS/MS using ACQUITY™ I-Class UPLC System coupled with Xevo™ TQ-XS Mass Spectrometer. We optimized a two-step protocol whereby the QuEChERS extract was pushed through the cartridge, the first milliliter discarded, and the remaining eluate collected for LC-MS/MS. The performance of the method has been successfully evaluated at 0.01 and 0.1 mg/kg in tea and cocoa, with most of the 395 analytes exhibiting recovery and repeatability within the tolerances set in the SANTE guidelines: 70–120% and ≤20% RSD, respectively. A simple pass-through SPE protocol with an Oasis PRiME HLB cartridge was proven to be a quick but effective alternative to dilute and shoot or dispersive SPE and has been shown to be suitable for checking MRL compliance for pesticide residues in tea and cocoa.
Benefits
- Pass-through cleanup with the Oasis PRiME HLB cartridge is an effective and quick means for removal of fats, phospholipids, and pigments from QuEChERS extracts whilst maintaining excellent recoveries for the pesticides of interest
- The performance of the method has been successfully evaluated using the SANTE acceptance criteria
- This method has been demonstrated as suitable for analysis of tea and cocoa, for checking compliance with MRLs and has the potential for determination at much lower concentrations
Introduction
Reliable analytical methods are needed for detection, quantification, and identification of hundreds of pesticide residues in many different commodities. National competent authorities are responsible for enforcement of agri-food chain legislation including sampling and analysis of agricultural commodities and foodstuffs to check compliance with maximum residue levels (MRLs). All food business operators must also ensure compliance with the same requirements but also must consider brand protection too. The analysis of the complex matrices, tea, and cocoa, is challenging due to the high amounts of endogenous components, such as fats, pigments, and other phytochemicals.1,2 These components are known to cause matrix effects and isobaric interference, which negatively impact detection and quantitation of the pesticide residues. Reducing the level of co-extractives by using an efficient clean-up, such as solid-phase extraction (SPE), with a simple pass-through protocol, helps to obtain reliable results, maintains instrument robustness, and minimizes the need for cleaning the system.
The objective of this work, conducted in collaboration with Galab Laboratories GmbH in Hamburg, was to establish the performance of a method based upon QuEChERS but using a simple pass-through SPE cleanup protocol with Oasis PRiME HLB, prior to UPLC-MS/MS using ACQUITY UPLC coupled with Xevo TQ-XS.
Experimental
Samples were homogenized, extracted, and analyzed by GALAB, using a modification of the QuEChERS CEN Method 156624.3 The optional dispersive SPE step was replaced by a simple pass-through SPE protocol with Oasis PRiME HLB in the Short Plus format, optimized for this analysis, prior to UPLC-MS/MS using ACQUITY UPLC coupled with Xevo TQ-XS (see Figure 1). The details of the LC-MS/MS method are proprietary to GALAB, but similar to that previous published by Waters.4 Validation of a method for 395 analytes was conducted in both commodities at 0.01 and 0.1 mg/kg, using the SANTE guidelines.5
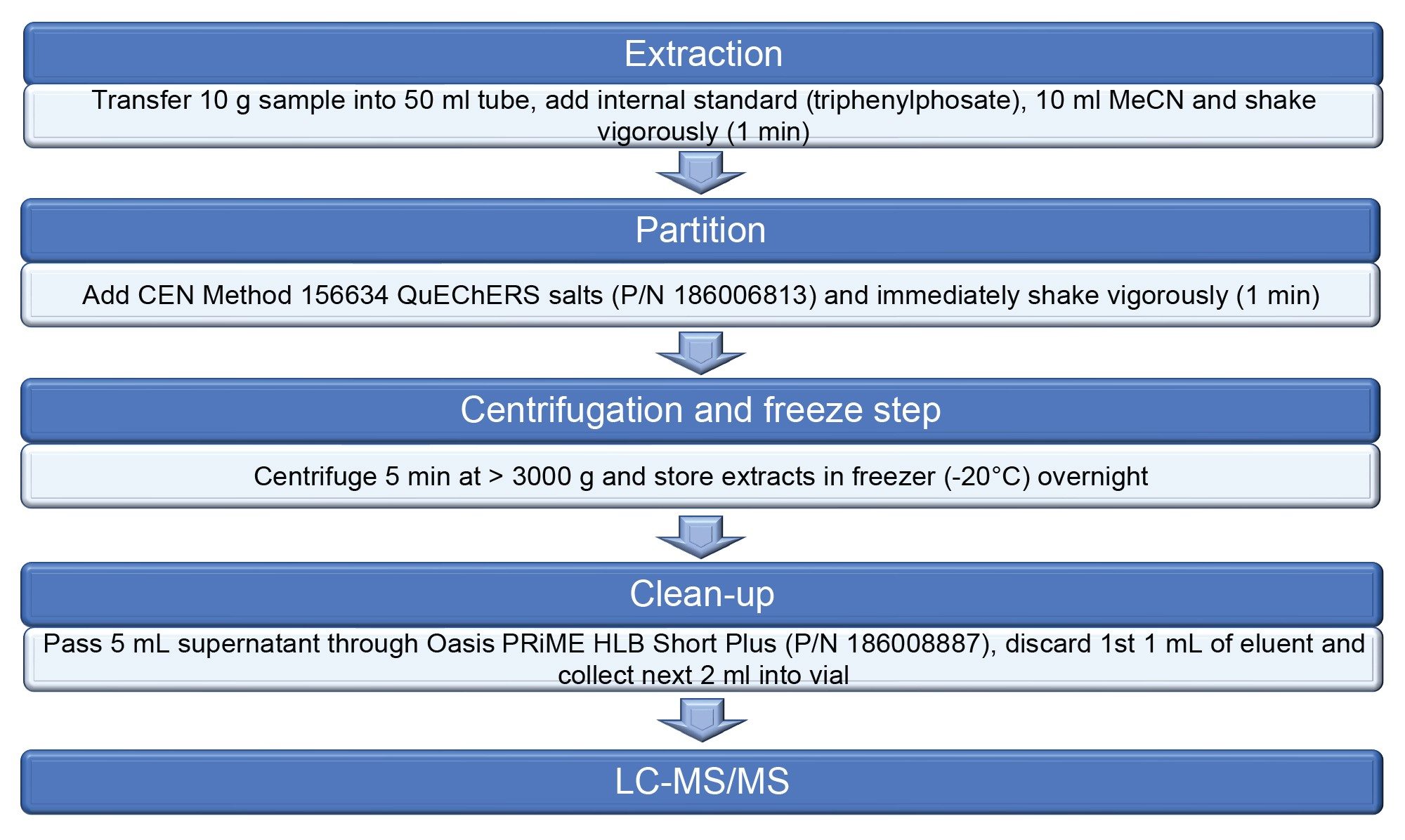
Results and Discussion
Chromatography was shown to be stable with no significant change in retention times across the batches analyzed. The ACQUITY UPLC BEH Phenyl Column provided sufficient retention for all but the most polar analytes and resulted in good separation and distribution of the 395 analytes within a 43-minute run time. Despite the large number of compounds, data quality, in terms of a sufficient number of data points across each peak, was not compromised.
Excellent sensitivity was demonstrated from the analysis of the matrix-matched standards prepared from tea and cocoa extracts. Figure 2 shows typical chromatograms for a selection of pesticides from the analysis of matrix-matched standards at 0.01 mg/kg in tea. Previous work had shown the response to be linear over the range required for this evaluation.4 To determine the concentration of analytes in the spikes, calibration graphs were created from the analysis of the two matrix-matched standards, using a linear fit and triphenyl phosphate as an internal standard. The selectivity of the analytical method was determined by analyzing blank tea and cocoa extracts. No significant interferences were detected at the same retention times of the analytes, but residues were detected for the biocides BACs 12 and 14, DDAC 10, and cetrimonium chloride, but this did not comprise the determination of recovery and repeatability (RSDr) as the same blank was used for spiking.
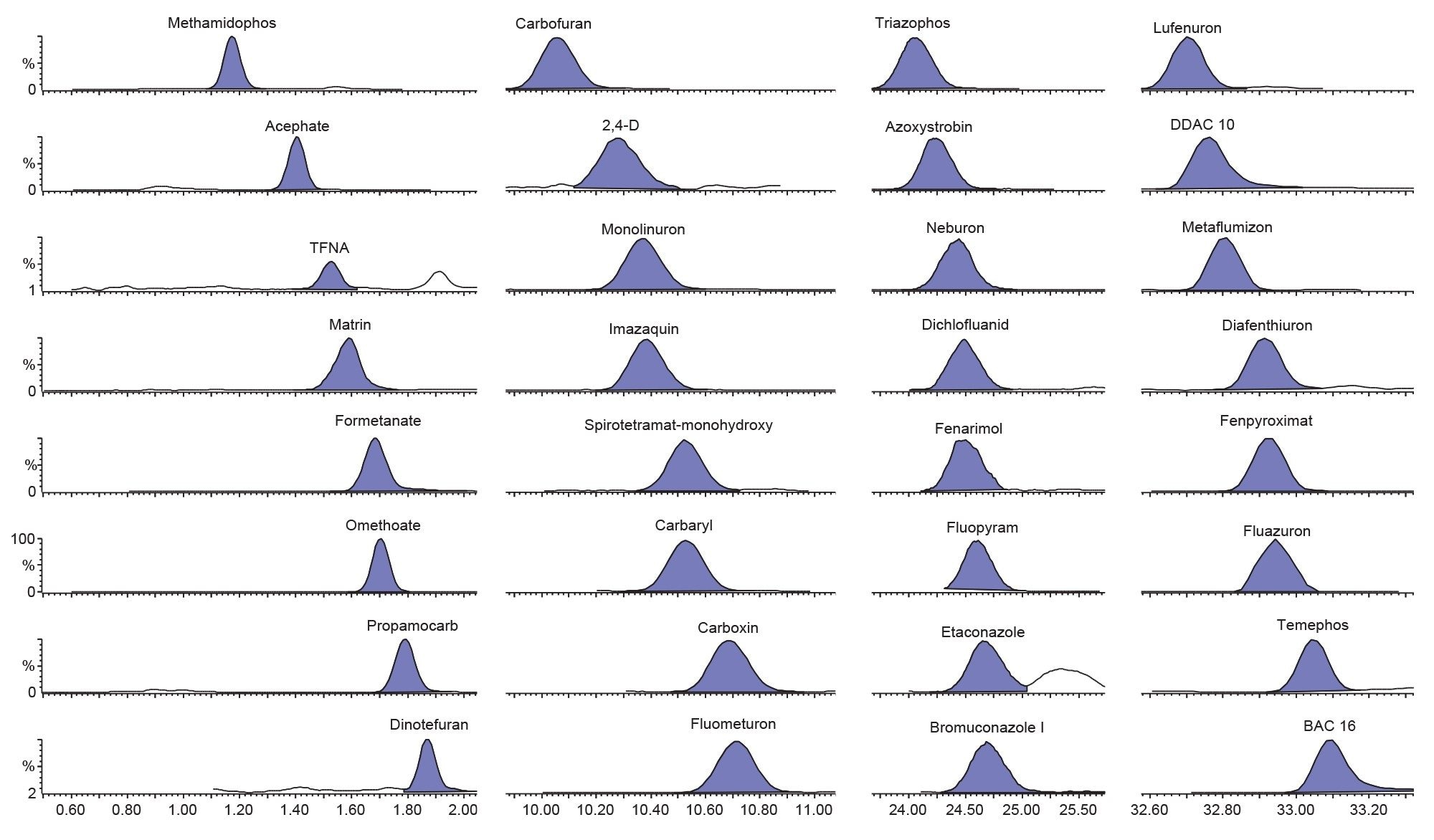
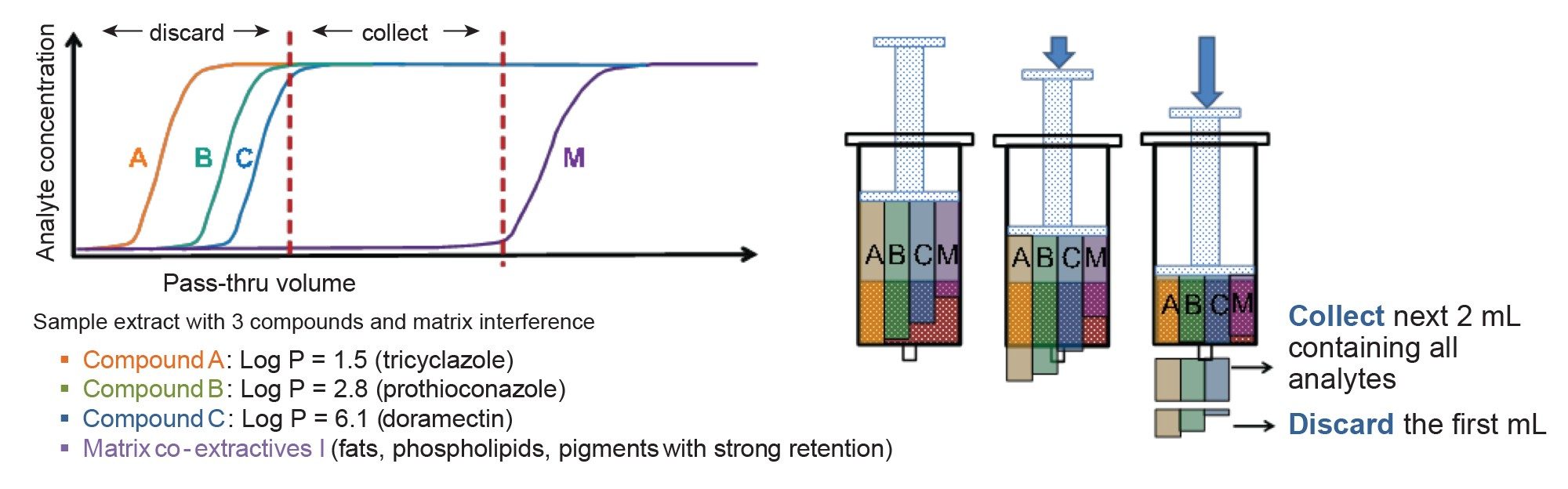
Dispersive SPE with multiple sorbents can be used to remove matrix co-extractives during QuEChERS but can lead to losses of certain pesticides. The pass-through SPE protocol inverts the classical SPE approach by passing through the analytes of interest and retaining co-extracted fats, phospholipids, and pigments on the sorbent. The Short Plus format of the Oasis PRiME HLB cartridge allows for extracts to be pushed through the cartridge manually using a syringe so that an eluate is obtained in only a few minutes ready for LC injection.5
The Oasis PriME Short Plus cartridge behaves as a short chromatography column. Clean-up of the extracted sample was optimized by evaluating the stage at which the analytes pass through the cartridge. Here, the first 1 mL that elutes was discarded before collecting most of the analytes with satisfactory recovery, whilst effectively removing matrix co-extractives (see Figure 3).
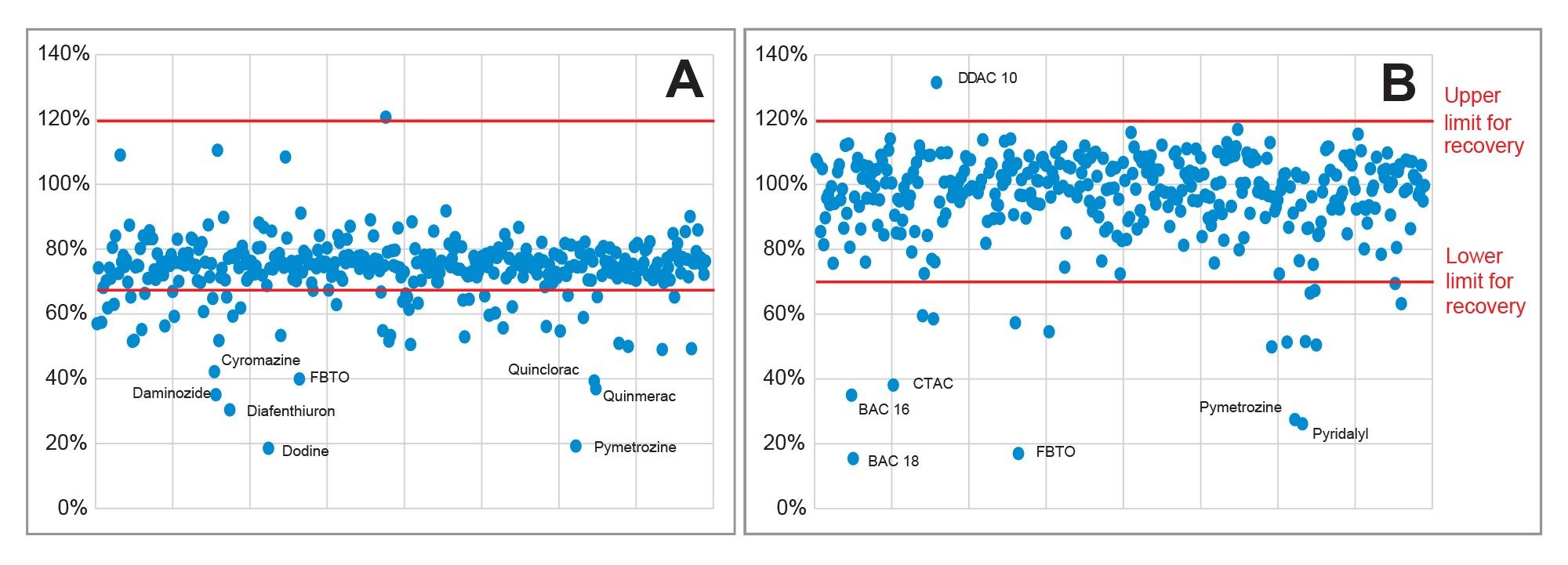
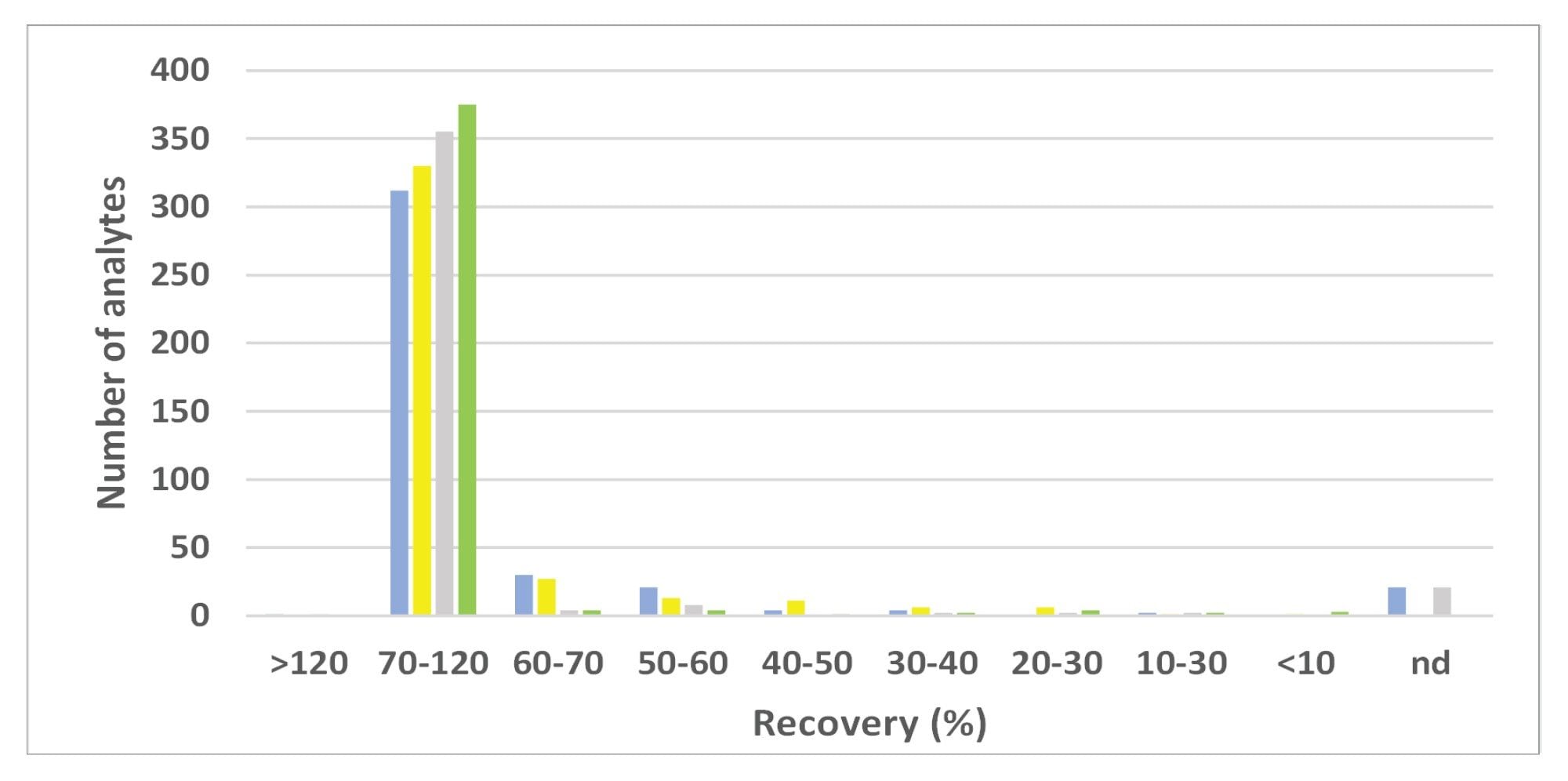
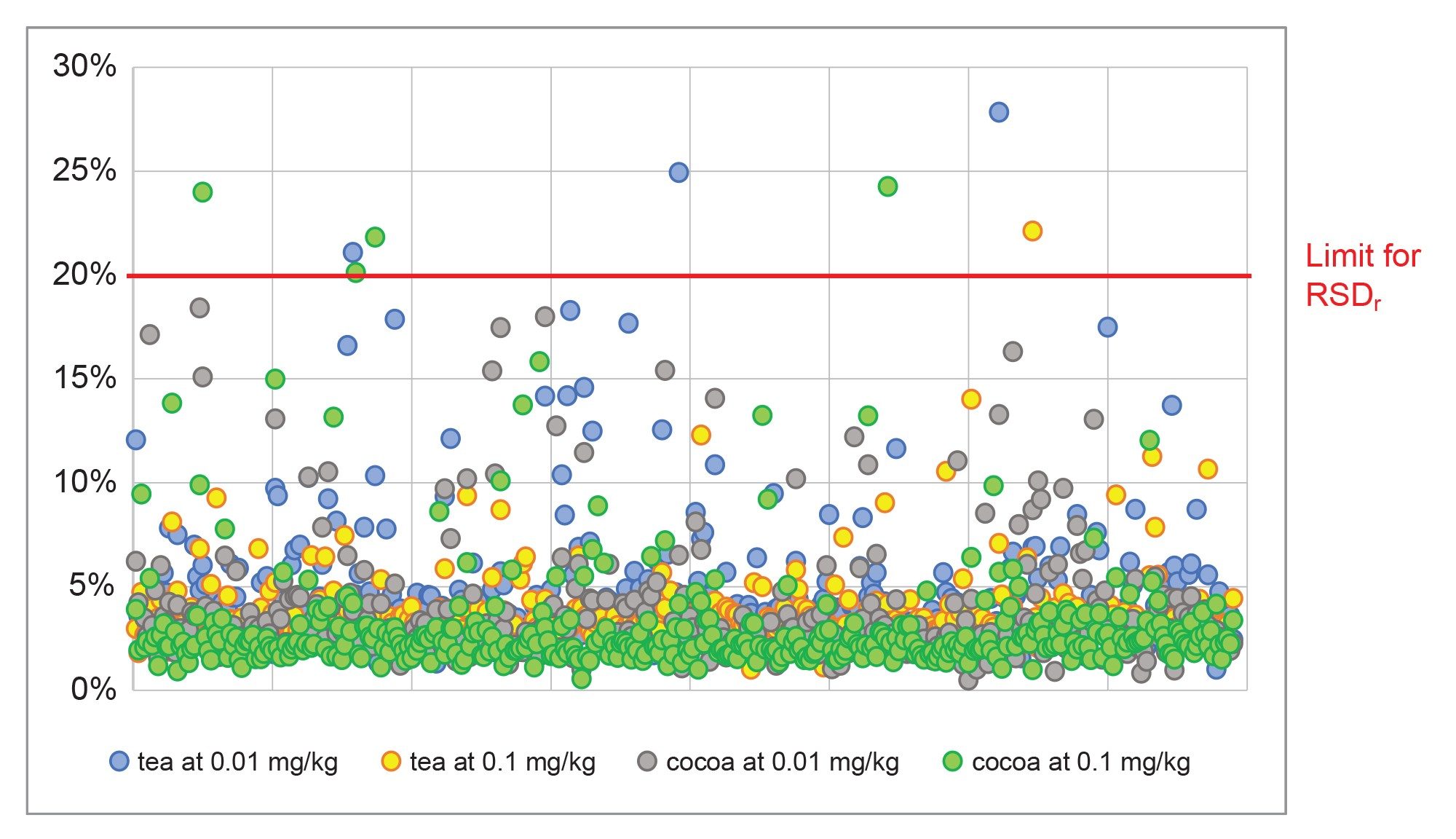
The SANTE guidelines specify an average recovery for each spike level tested to be between 70 and 120%.5 The results from analysis of the spikes at 0.01 mg/kg in tea and cocoa are shown in Figure 4. The majority of the 395 analytes were within tolerance for both commodities at 0.01 mg/kg: 79% in tea and 90% in cocoa. The actual values for recovery from tea were somewhat lower than that obtained from cocoa. Some analytes (5% of the 395) were not detected at 0.01 mg/kg. The identity of some of the outliers is annotated in the figure. At the higher concentration, 0.1 mg/kg, 84%, and 90% of the analytes were within the tolerance in tea and cocoa, respectively. Figure 5 shows the distribution of all measured recoveries, in both commodities, at both concentrations. Figure 6 shows the distribution of RSDr (one outlier removed). The SANTE guidelines recommend that RSDr for each spike level tested should be ≤20%. Excluding the non-detects at 0.01 mg/kg, 99% of the analytes from all the batches tested were within the tolerance specified. Almost all the compounds exhibiting recoveries between 30 and 140% showed consistent results with RSDr ≤20%.
Conclusion
This application note has described a sensitive multiresidue method for the determination of pesticide residues in two complex food commodities, tea, and cocoa, using UPLC-MS/MS (Xevo TQ-XS). A simple pass-through SPE protocol with Oasis PRiME HLB has proven to be a quick but effective alternative to dilute and shoot or dSPE. The performance of the method has been successfully evaluated using the SANTE acceptance criteria, with most analytes exhibiting recovery and RSDr within the tolerances set: 70–120% for recovery and ≤20% for RSDr. This method has been shown to be suitable for determination of pesticide residues in tea and cocoa, for checking compliance with EU MRLs, and has the potential for determination at much lower concentrations.
References
- Ly T-K et al. Quantification of 397 Pesticide Residues in Different Types of Commercial Teas: Validation of High Accuracy Methods and Quality Assessment. Food Chem. (2022) 370:130986.
- Comprehensive Strategy for Pesticide Residue Analysis in Cocoa Beans Through Qualitative and Quantitative Approach. Food Chem. (2022) 368:130778.
- European Committee for Standardisation (CEN) EN 15662:2018. Foods of Plant Origin - Multimethod for the Determination of Pesticide Residues Using GC- And LC- Based Analysis Following Acetonitrile Extraction/Partitioning and Clean-up by Dispersive SPE - Modular QuEChERS-Method.
- Shah D., Wood J., Fujimoto G., McCall E., Hird S., Hancock P. Multiresidue Method for the Quantification of Pesticides in Fruits, Vegetables, Cereals and Black Tea using UPLC-MS/MS. Waters Application Note. 720006886. 2020.
- Young M., Shia J. Oasis PRiME HLB Cartridges Now Available in Syringe Compatible Plus Format. Waters Application Note. 720006017. 2017.
- Document No. SANTE/11312/2021. Guidance Document on Analytical Quality, Control, and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. 2021.
720008039, September 2023