Part 1: Replicating an HPLC Method for Water-Soluble Vitamins on the Alliance™ iS HPLC System
Abstract
With the increasing popularity of vitamin supplements, there is a need to ensure that these products meet the content descriptions. High performance liquid chromatography (HPLC) is an essential analytical tool to ensure products meet label claims. However, the wide range of chemical characteristics makes analysis of vitamins with a single mode of chromatography challenging. For water-soluble vitamins, hydrophilic interaction chromatography (HILIC) allows for retention of highly polar vitamins.1 In addition, these vitamins exist within complex matrices, often making sample preparation challenging and laborious.
In regulated labs, the ability to migrate these methods to different HPLC systems is essential as systems are updated and replaced with newer systems. In this work, we will document the ability to move methods for water-soluble vitamins under gradient HILIC conditions across HPLC systems. Here, we will demonstrate the ability to achieve the same quantitative results on legacy and newer HPLC systems. The legacy Alliance e2695 System and the Alliance iS HPLC System were chosen for method migration. In Part 2 of this 2-part method migration study, replicating an HPLC method for fat-soluble vitamins on the Alliance iS HPLC System will be discussed.
Benefits
- Quantitation of water-soluble vitamins in multivitamin supplements using the Alliance iS HPLC System
- Easy method transfer from legacy HPLC systems to the Alliance iS HPLC System
Introduction
Vitamin analysis is performed regularly in a wide array of laboratories. For dietary supplement laboratories, it is useful to test samples throughout the production process to ensure that ingredients and finished products have the expected concentration of desired vitamins. In the United States, 21 CFR 101.9(g)(4) indicates that added Class I ingredients must be at least 100% of the declared value on the label, with consideration of analytical method variation.2 Products with labels that do not meet this requirement are considered misbranded, which further justifies the significance of analytical testing.
When performing vitamin analysis, sample preparation can be difficult as matrix interferences may be present in some multivitamin formulations. At times, this can be mitigated with additional sample dilutions, solid phase extraction techniques or liquid-liquid extractions. For more complex matrices, additional sample cleanup or multiple extractions may be required.3
In addition to the different analytical challenges that vitamins pose naturally, within a typical analytical laboratory, there are a variety of HPLC systems that can be used for analysis. Furthermore, as labs update their instrumentation, there may be a need to take advantage of modern technologies. These factors make it important to be able to migrate methods across different systems, with each system producing the same quantitative results for a given sample.
This work will demonstrate the migration of a multivitamin analysis from a legacy HPLC system, specifically the Alliance e2695 HPLC System, to an Alliance iS HPLC System. Comparable quantitative results between the two systems will be shown.
Experimental
Water-Soluble Standard Preparation
Nicotinamide, pyridoxal, nicotinic acid, riboflavin, thiamine, vitamin B12, and folic acid standards were prepared using standards purchased from Sigma-Aldrich. Two separate stock solutions were prepared. Stock solution 1 contained riboflavin at a concentration of 1 mg/mL and vitamin B12 and folic acid at a concentration of 2 mg/mL in DMSO. Stock solution 2 contained pyridoxal at a concentration of 1 mg/mL and nicotinamide, nicotinic acid, and thiamine at a concentration of 2 mg/mL in 20:80 acetonitrile:water. A combined stock solution was prepared at a concentration of 50 µg/mL for nicotinamide, nicotinic acid, riboflavin, thiamine, vitamin B12, and folic acid, and at a concentration of 25 µg/mL for pyridoxal and riboflavin, in 20:80 acetonitrile:water. The stock solution was further diluted using 20:80 acetonitrile:water to yield calibration standards at 1.57, 3.13, 6.25, 12.5, 25, and 50 µg/mL for vitamin B12 and folic acid, which were the vitamins present in the multivitamin supplement sample.
Note: Amber glassware was used to prevent degradation of vitamin B12 and folic acid which are light-sensitive.
Water-Soluble Multivitamin Supplement Sample Preparation
The multivitamin supplement sample was prepared by dissolving ten tablets into 25 mL of water in a 50 mL centrifuge tube. The samples were shaken for 30 minutes followed by centrifugation at 3900 rpm for 10 minutes. Ten (10) mL of the supernatant was transferred to a clean 50 mL centrifuge tube and diluted 1:1 with acetonitrile. Additional sample dilutions using 20:80 water:acetonitrile were required for quantitation.
LC Conditions
|
LC systems: |
Alliance e2695 HPLC System and Alliance iS HPLC System |
|
Detection: |
TUV Detector with 10 mm HPLC flow cell |
|
Wavelength: |
265 nm |
|
Sampling rate: |
2 Hz |
|
Vials: |
LCGC certified clear glass 12 x 32 mm screw neck vial, total recovery with cap and Preslit PTFE/Silicone septum 2 mL Volume (p/n: 186000307C) |
|
Column(s): |
XBridge™ Amide Column, 3.5 µm, 4.6 x 250 mm (p/n: 186004870) |
|
Column temperature: |
30 °C |
|
Sample temperature: |
15 °C |
|
Injection volume: |
25 µL |
|
Flow rate: |
1.0 mL/min |
|
Mobile phase A: |
100 mM ammonium acetate, pH=5.5 |
|
Mobile phase B: |
Acetonitrile |
|
Mobile phase C: |
Water |
|
Sample manager wash: |
50:50 (v/v) Water:Acetonitrile |
|
Sample manager purge: |
20:80 (v/v) Water:Acetonitrile |
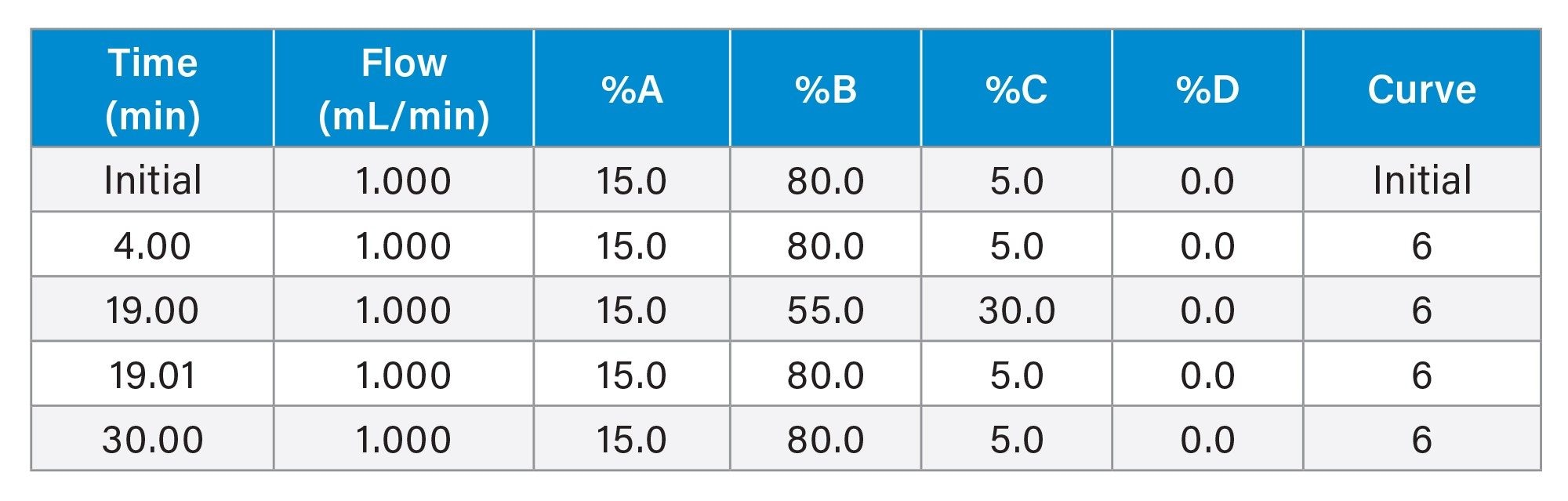
Gradient Table
Data Management
|
Chromatography data system: |
Empower™ 3, FR 3.7.0 |
Results and Discussion
Water-Soluble Vitamins Method Migration
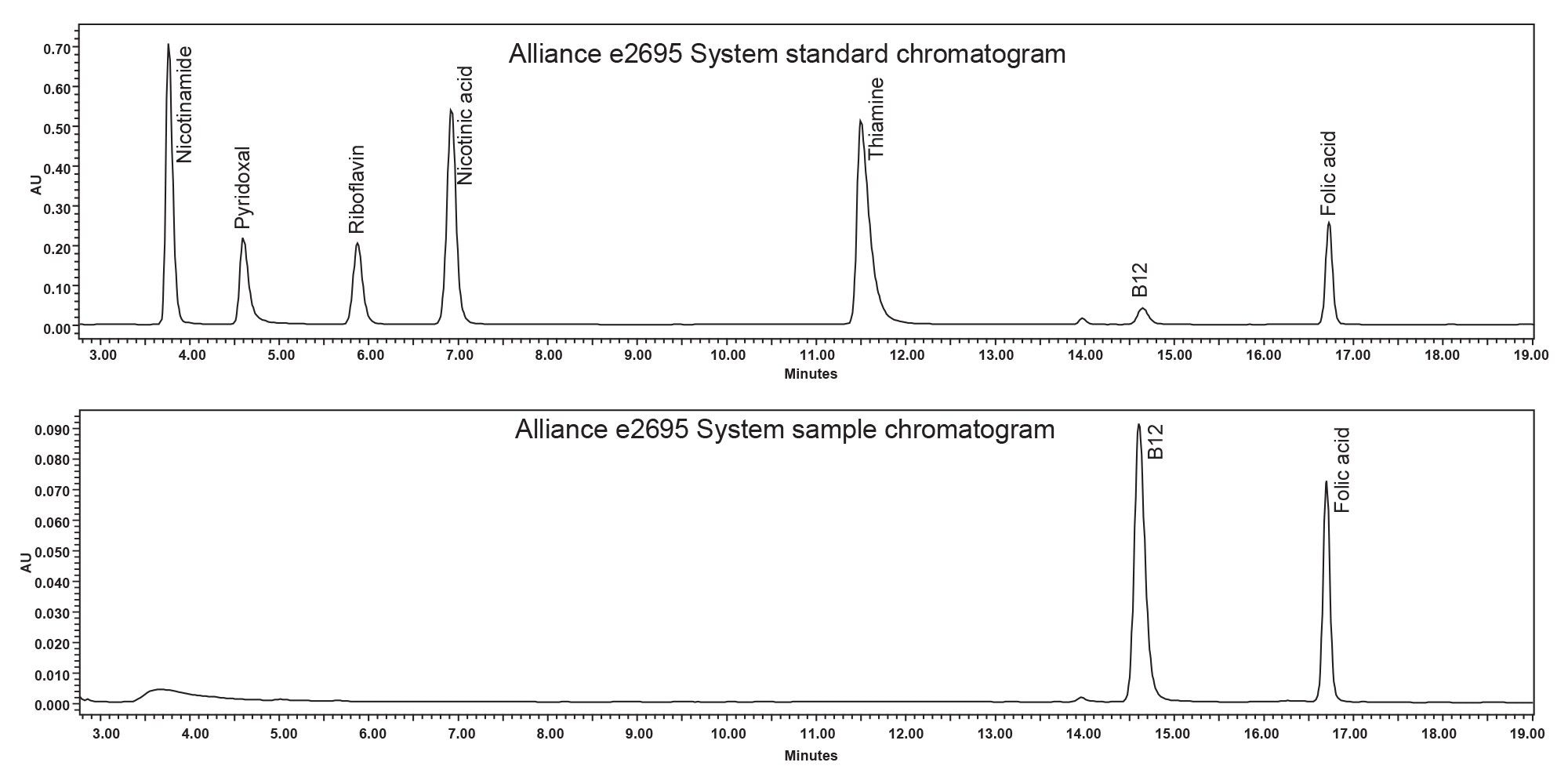
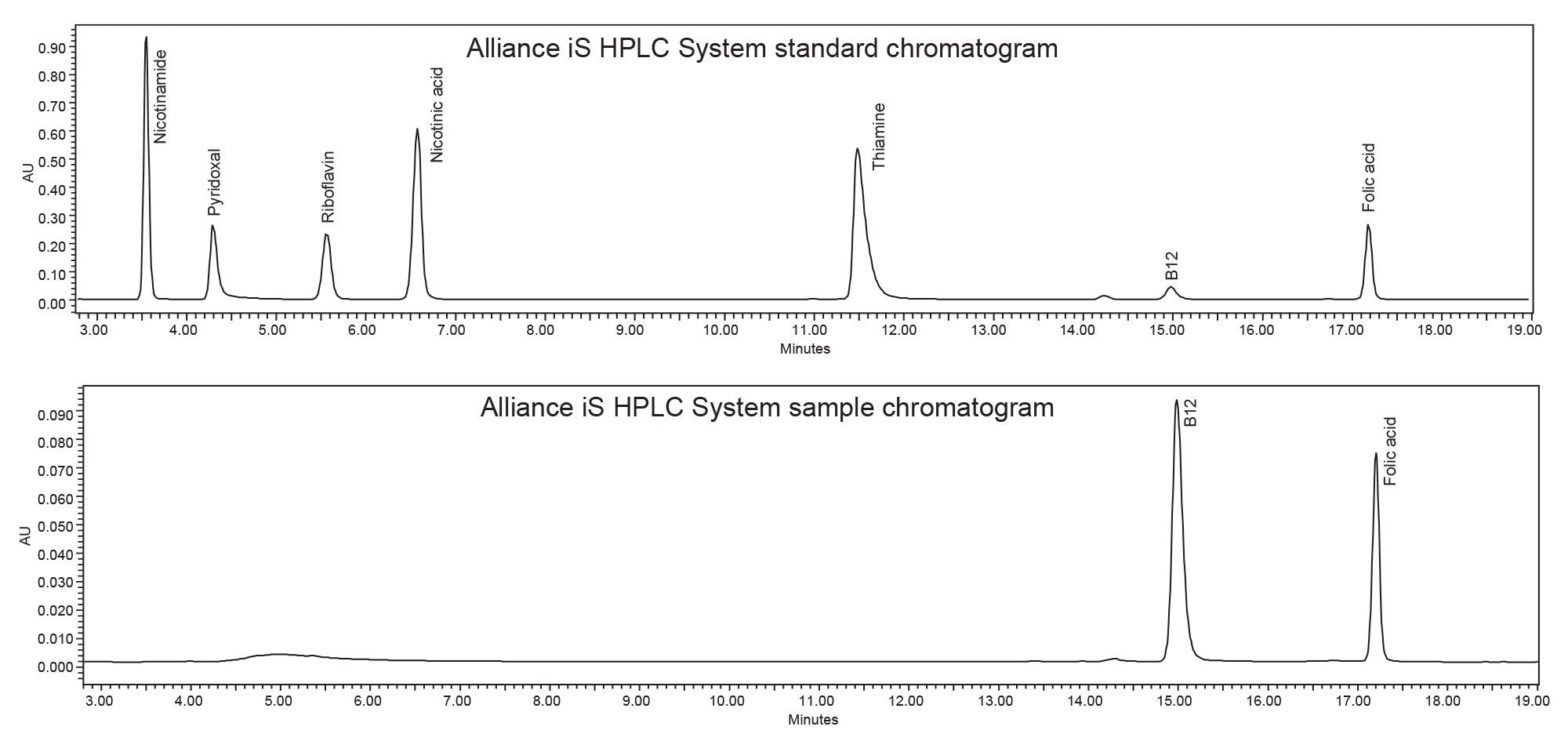
Analysis of vitamins in supplements is critical to ensure product safety and accuracy of the product’s label claim. A multivitamin sample containing both vitamin B12 and folic acid was analyzed on the Alliance e2695 and the Alliance iS HPLC System. The analysis on each system used the same preparation of mobile phase, standard, and sample, as well as the same chromatographic column. The results from both systems were compared to the label claim of the multivitamin supplement sample along with a comparison of the results between the two systems. Example chromatograms of the vitamin standard and the multivitamin supplement sample for both systems are shown in Figures 1 and 2.
The method was successfully migrated from the Alliance e2695 System to the Alliance iS HPLC System, as all the system suitability criteria were met without additional method adjustments. The systems showed a slight shift in retention time. The Alliance e2695 System had retention times slightly earlier than the Alliance iS HPLC System, which can be seen in Figures 1 and 2. The delay volume differences between the two systems can contribute to this retention time shift.
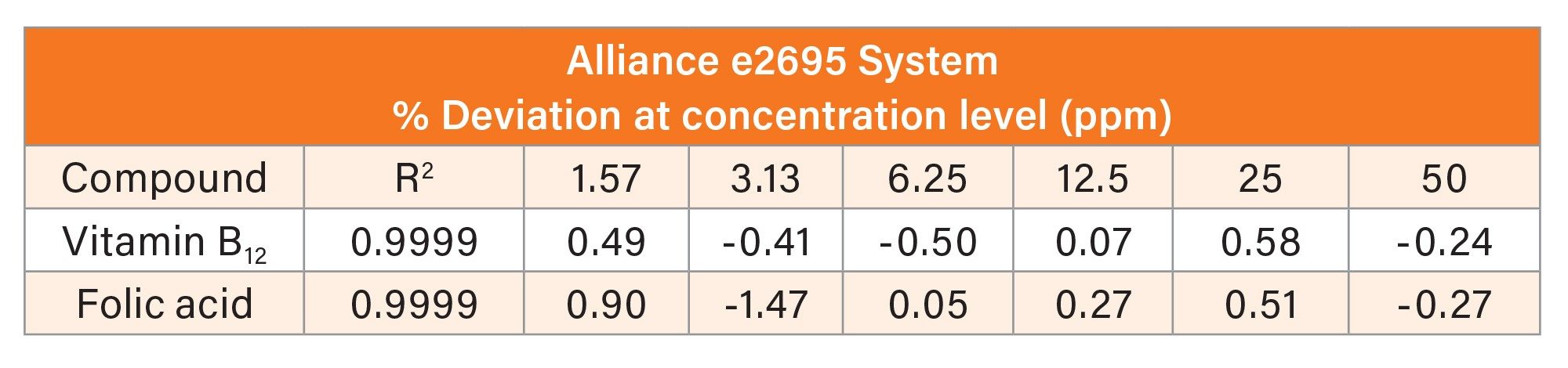
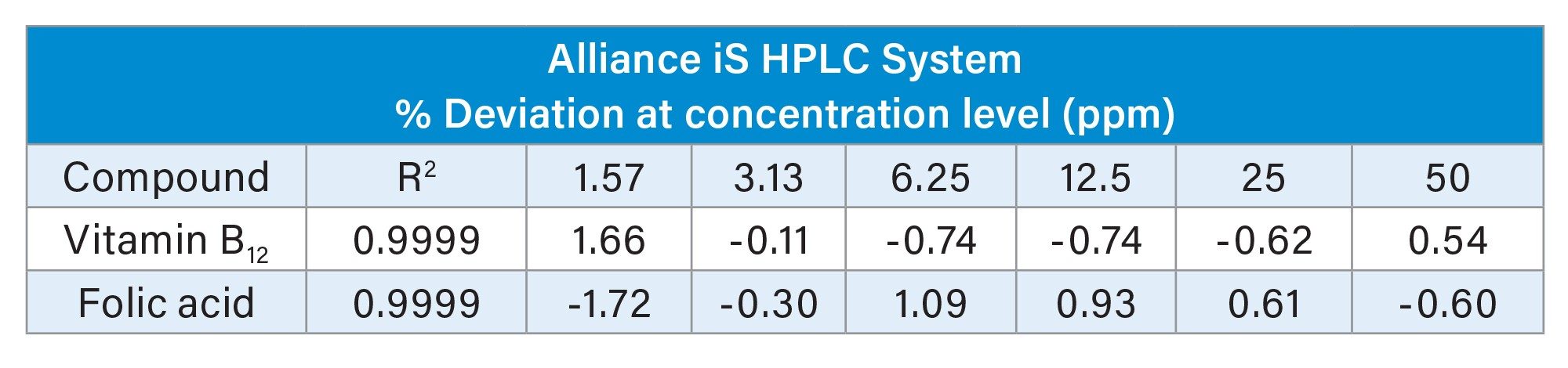
The % deviation from nominal concentration and R2 results for both systems are shown in Table 1. The calibration curve R2 values were 0.9999 and the % deviation from nominal concentration was within ± 1.75 for both systems.
Water-Soluble Vitamin System Suitability and Supplement Sample Results
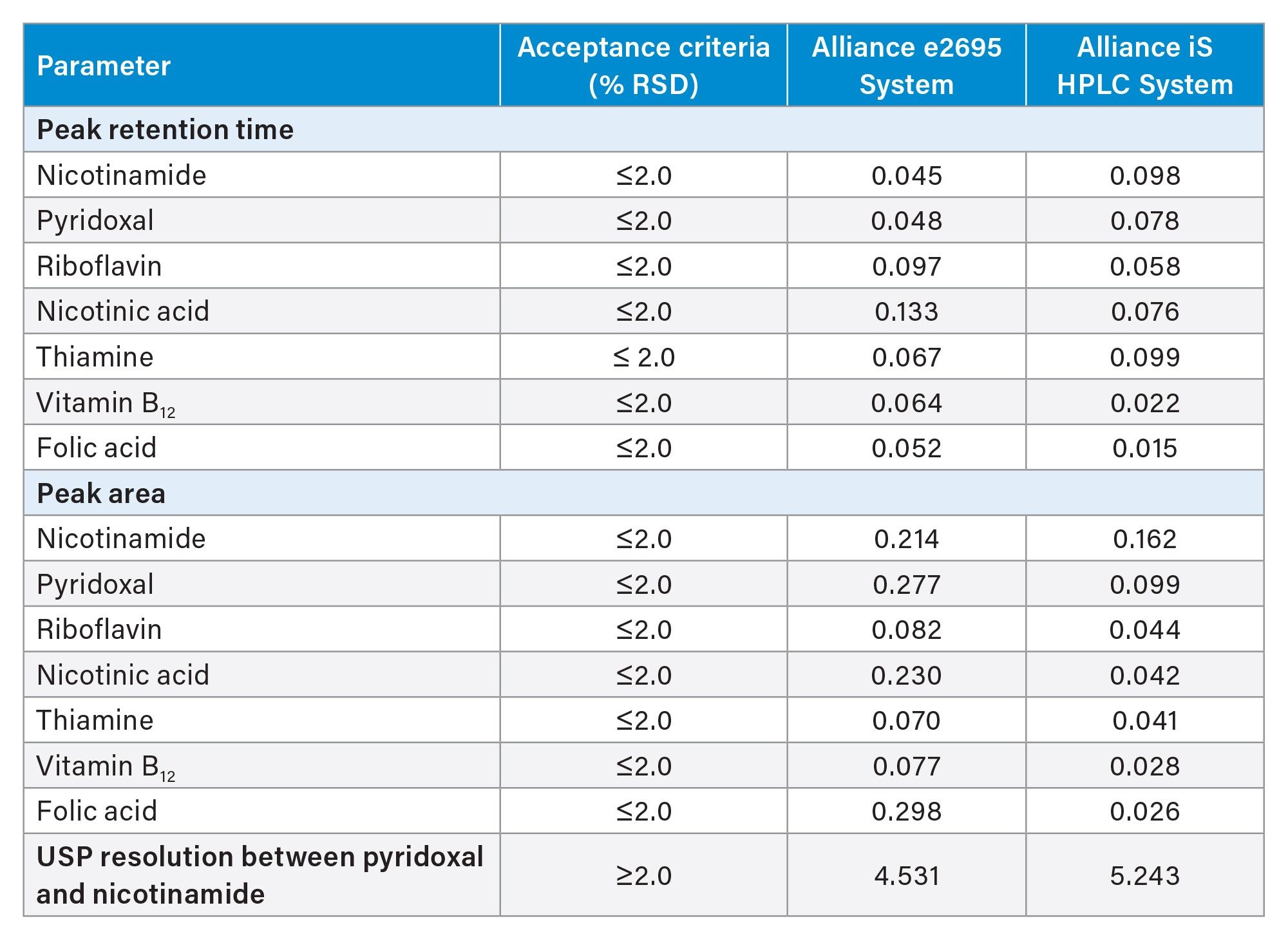
System suitability was determined by making five replicate injections of the working standard preparation and calculating the retention time and area %RSD of each of the seven standard compounds. The resolution between the pyridoxal and nicotinamide was also determined. The results from the Alliance e2695 System and the Alliance iS HPLC System are comparable and meet the established system suitability criteria of the method. The results are shown in Table 2.
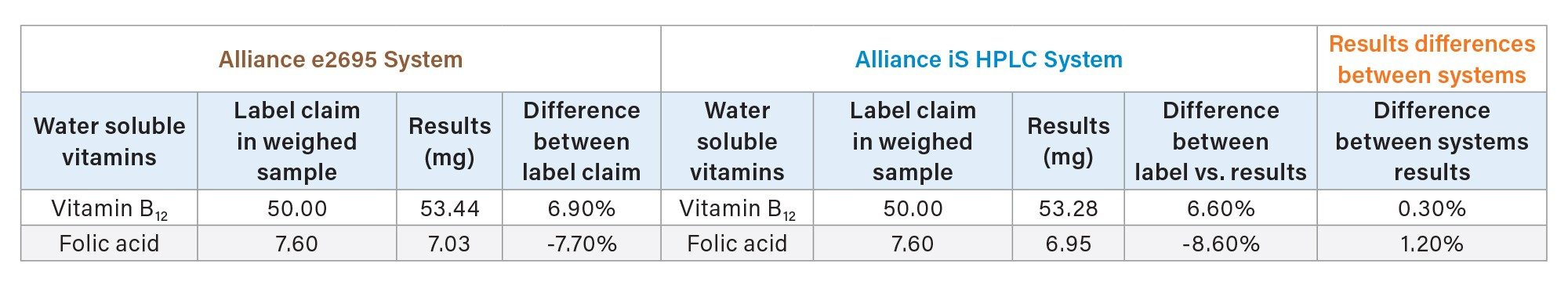
The multivitamin supplement tablet had a label claim of 50 mg per tablet for vitamin B12 and 7.6 mg per tablet for folic acid. The quantitative results for vitamin B12 and folic acid obtained using the Alliance e2695 and Alliance iS HPLC systems were compared to the label claims and are shown in Table 3. Both systems show good agreement with a results difference of 0.3% for vitamin B12 and 1.2% for folic acid.
Total content for vitamin B12 and folic acid in the multivitamin supplement sample was found to be within ± 9% of the label claim. These results demonstrate the ability to quantify vitamin B12 and folic acid in the multivitamin supplement sample within a commercially relevant concentration range using both the Alliance e2695 System and the Alliance iS HPLC System. Of note, the quantitative values for folic acid were less than the labeled values by 7.70–8.60%. Before determining a product to be misbranded, laboratories would need to fully validate their analytical method for the appropriate sample matrix and ensure that a representative sample was used.
Conclusion
Analysis of vitamin content in multivitamin supplements is critical to ensure accuracy of the product’s label claim. While analysis of multivitamin supplements can pose certain challenges, the method described here produced reproducible and reliable results on both the legacy Alliance e2695 and modern Alliance iS HPLC systems. The close agreement of quantitative results obtained on the systems, within 1.2%, demonstrates successful migration of a HILIC method to a modern HPLC system. Quantification of the multivitamin supplement showed good agreement for water-soluble vitamins, within ± 9% of the label claim.
References
- Eric S. Grumbach and Kenneth J. Fountain. Comprehensive Guide to HILIC Hydrophilic Interaction Chromatography. Waters Corporation, 2010.
- 21 CFR 101.9: Food Labeling – Nutrition Labeling of Food. (Current through 09/28/2023).
- Kim Tran and Peter Hancock. Analysis of Water-Soluble Vitamins and Caffeine in Beverage and Multivitamin Products by Arc HPLC System with PDA Detection, Waters Application Note, 720007357, 2021.
720008159, December 2023