Enhancing Size-Exclusion Chromatography of Large Biomolecular Analytes With Rigorously Designed GTxResolve™ Premier SEC 1000 Å 3 µm Columns
Abstract
This application note discusses the benefits of GTxResolve™ Premier SEC 1000 Å Columns for performing size exclusion chromatography of large biomolecules during their characterization. Robust SEC methods must demonstrate high reproducibility, minimal adsorption, and low secondary interactions of analytes, which are effects that can be attributed to column hardware and properties of the packing material. In this app note, we introduce GTxResolve™ Premier SEC 1000 Å Columns with MaxPeak™ High-Performance Surfaces (HPS), using novel ethylene bridged polyethylene oxide (HO-PEO) bonded 3 µm particle packing material with an average pore diameter of 1000 Å. This pore and particle size has been tailored specifically for large molecule analyses. These columns exhibit excellent inertness, high resolution, recovery, and reproducibility at both batch-to-batch and column-to-column levels using standard mobile phases, all of which is a step change in performance and reliability compared to commercially available 5 µm particle 1000 Å pore size columns. The resolution provided by these columns will make it possible to more quickly measure the process and product-related impurities present in cell and gene therapy products such that the industry can make quick progress toward more efficacious, safer, and more globally accessible medicines.
Benefits
- Novel ethylene bridged HO-PEO bonded 3 µm particles that provide improved chemical inertness and high efficiencies
- Excellent resolution for large biomolecules, including high molecular weight protein complexes, and nucleic acids
- Reproducibility confirmed by quality control batch testing with two molecule types - both protein and nucleic acid-based samples
- Enhanced chromatographic performance manifest as higher signal, sharper peaks, and more plates
- Minimal adsorption and significantly reduced secondary interactions to the column matrix to facilitate more robust results with more mobile phase compositions
Introduction
Size exclusion chromatography (SEC), also called gel permeation, gel filtration, steric exclusion, or just gel chromatography, is a chromatographic technique that separates analytes based on the relative size or hydrodynamic volume of macromolecules in accordance with the average pore size of the packing material.1 It has become a widely accepted and well-established separation technique over the years and has played significant roles in characterizing drug substances for their critical quality attributes.2 However, the analysis of cell and gene therapy products such as protein complexes, mRNA, and lipid nanoparticles require high pore volumes, particle surface chemistries with improved inertness, and an overall reduction in secondary interactions. In addition, new levels of throughput are needed in the analytical testing of cell and gene therapy drug products, so it would be valuable to develop SEC columns with more efficient, smaller particle size packing materials.
Macromolecular biomolecules are known to be susceptible to nonideal secondary interactions with traditional packing materials and column hardware surfaces owing to the presence of concentrated electrostatic patches and regions of accessible hydrophobic residues. These secondary interactions can lead to shifts in elution times, low recovery, poor peak shapes, and peak tailing.3 Even though electrostatic interactions can be minimized by increasing the ionic strength, higher salt concentrations can be problematic for downstream detection techniques such as mass spectrometry or, in some cases, keeping the molecular complexes intact. Similarly, organic solvent can be added to an SEC mobile phase to lessen hydrophobic interactions, but this can risk denaturing the delicately folded biomolecules, especially when organic concentrations greater than 5% are needed.
This application note highlights the enhanced chromatography that can be obtained with the adoption of GTxResolve Premier SEC 1000 Å 3 µm Columns. Performance improvements include enhanced resolution, efficiency, improved signal, batch-to-batch and column-to-column reproducibility, minimal adsorption, and very little or no secondary interactions as compared to the currently available 5 µm silica particle columns of similar pore size.
Experimental
Sample Preparation
2X Strength PBS Buffer (20 mM Phosphate, 276 mM NaCl, 5.4 mM KCl pH 7.4): 2 packets of Sigma Phosphate Buffered Saline (Sigma p/n: P-3813) was dissolved in 1 liter of 18.2 MΩ water and filtered through a 0.2 µm filter.
200 mM Sodium Phosphate Buffer pH 6.8: 28.39 g of sodium phosphate dibasic anhydrous was dissolved in 1 liter of 18.2 MΩ water and the pH adjusted to 6.8 using concentrated HCl.
Protein Standard Mix: Waters™ BEH™ 450 SEC Protein Standard Mix (p/n:186006842) was used as the protein standard. This standard is a five component protein mixture consisting of 0.1 mg/mL Thyroglobulin dimer, 3 mg/mL Thyroglobulin monomer, 2 mg/mL IgG, 5 mg/mL BSA, 2 mg/mL Myoglobin, and 0.1 mg/mL Uracil. The lyophilized standard was reconstituted using 1 ml of the 200 mM Sodium Phosphate pH 6.8 Buffer before use.
dsDNA 50 to 1350 Ladder: Waters dsDNA 50 to 1350 Ladder (p/n:186010778), a lyophilized mixture of 17 double-stranded DNA species ranging from 50 bp to 1,350 bp was used as the DNA standard. This lyophilized standard was reconstituted using 100 µL of 2x PBS Buffer.
Ado-trastuzumab emtansine (Kadcyla™ (ado-trastuzumab ematansine)) (Antibody Drug Conjugate): 5 mg/mL, diluted from 20 mg/mL stock using 18.2 MΩ water.
NIST Monoclonal Antibody Reference Material (NISTmAb): 2 mg/mL, diluted from 10 mg/mL stock using Histidine buffer (12.5 mM Histidine in 12.5 mM Histidine-HCl).
LC Conditions for Reproducibility Studies
|
LC system: |
ACQUITY™ UPLC™ H-Class Bio System (Equivalent to an ACQUITY Premier System with a Quaternary Solvent Manager and High pH Kit) |
|
Detection: |
ACQUITY TUV Detector |
|
Wavelength: |
260/280 nm |
|
Vials: |
Polypropylene 12 x 32 mm Screw Neck Vial, with Polyethylene Septum-less Cap, 300 µL Volume (p/n: 186004112) |
|
Column(s): |
GTxResolve Premier SEC 1000 Å 3 µm 4.6 x 150 mm Column (p/n: 186010735); GTxResolve Premier SEC 1000 Å 3 µm 4.6 x 300 mm Column (p/n: 186010736) |
|
Column temperature: |
35 °C |
|
Sample temperature: |
6 °C |
|
Injection volume: |
3.5 µL or 20 µL (Protein Mix), 5 µL (dsDNA 50 to 1350 Ladder) |
|
Flow rate: |
0.1 mL/min for 150 mm, 0.2 mL/min for 300 mm columns |
|
Mobile phase A: |
2X PBS Buffer (20mM Phosphate, 276 mM NaCl, 5.4 mM KCl pH 7.4) |
|
Mobile phase B: |
18.2 MΩ·cm Water |
|
Mobile phase C: |
Column storage solution (10% acetonitrile in 25mM Sodium Phosphate + 100mM KCl solution) |
|
Mobile phase D: |
18.2 MΩ·cm Water |
|
Gradient: |
Please refer to the table below. |
LC Conditions to Test for Secondary Interaction
|
LC system: |
ACQUITY™ UPLC™ H-Class Bio System (Equivalent to an ACQUITY Premier System with a Quaternary Solvent Manager and High pH Kit) |
|
Detection: |
ACQUITY TUV Detector |
|
Wavelength: |
260/280 nm |
|
Vials: |
Polypropylene 12 x 32 mm Screw Neck Vial (p/n: 186002639), with Polyethylene Septumless Cap, 300 µL Volume (p/n: 186004112) |
|
Column(s): |
GTxResolve™ Premier SEC 1000 Å 3 µm 4.6 x 150 mm Column (p/n: 186010735) |
|
Column temp.: |
35 °C |
|
Sample temp.: |
6 °C |
|
Injection volume: |
2 µL (Protein Mix), 1 µL (NISTmAb, Kadcyla) |
|
Flow rate: |
0.25 mL/min |
|
Mobile phase A: |
200 mM sodium phosphate pH 6.8 |
|
Mobile phase B: |
1 M NaCl |
|
Mobile phase C: |
50:50 Acetonitrile: 18.2 MΩ water [v/v]) |
|
Mobile phase D: |
18.2 MΩ·cm Water |
|
Gradient: |
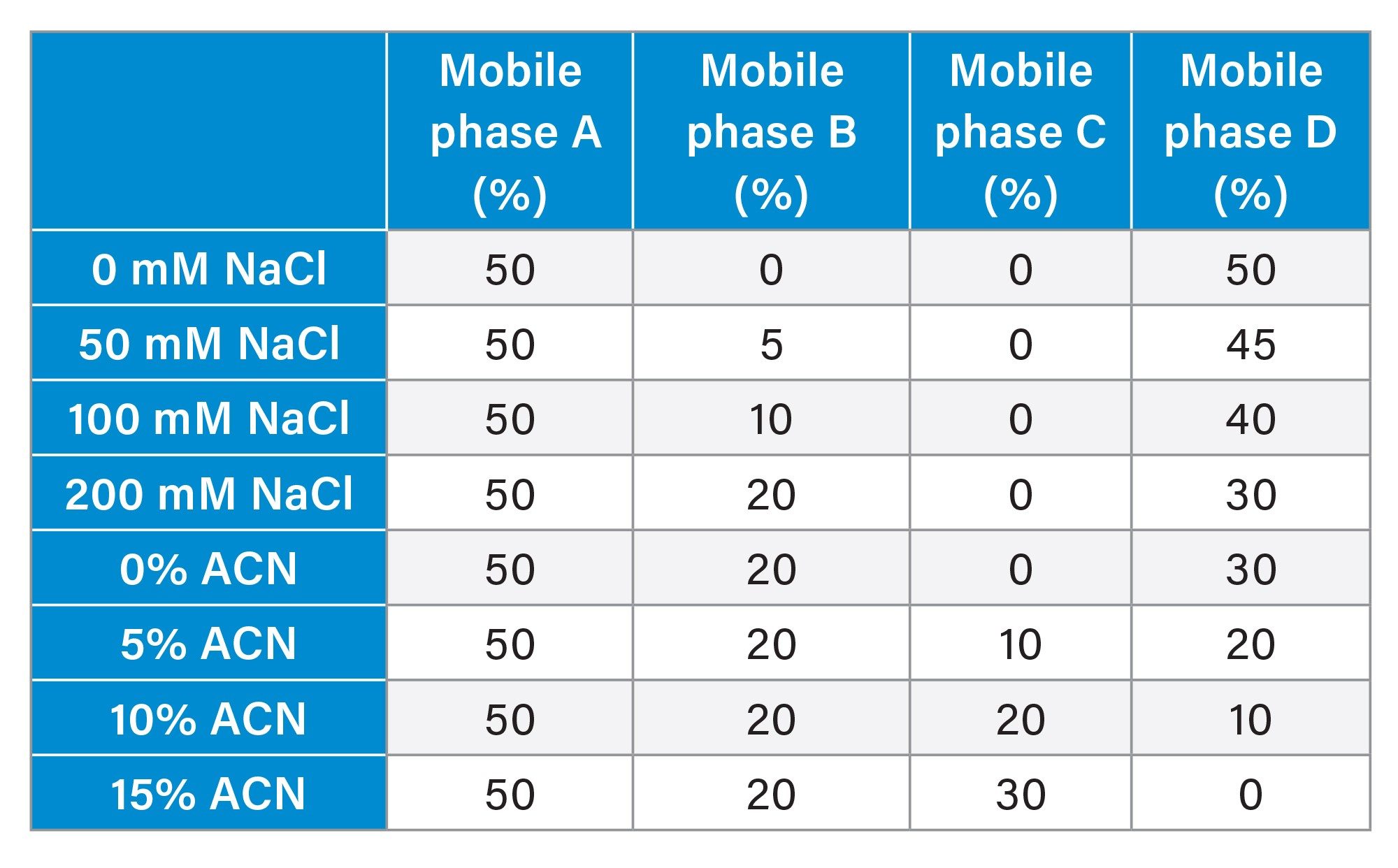
Isocratic runs. Please refer to the table below for mobile phase compositions for various test conditions. |
Gradient table
Results and Discussion
GTxResolve Premier SEC 1000 Å 3 µm Particles and Column Architecture
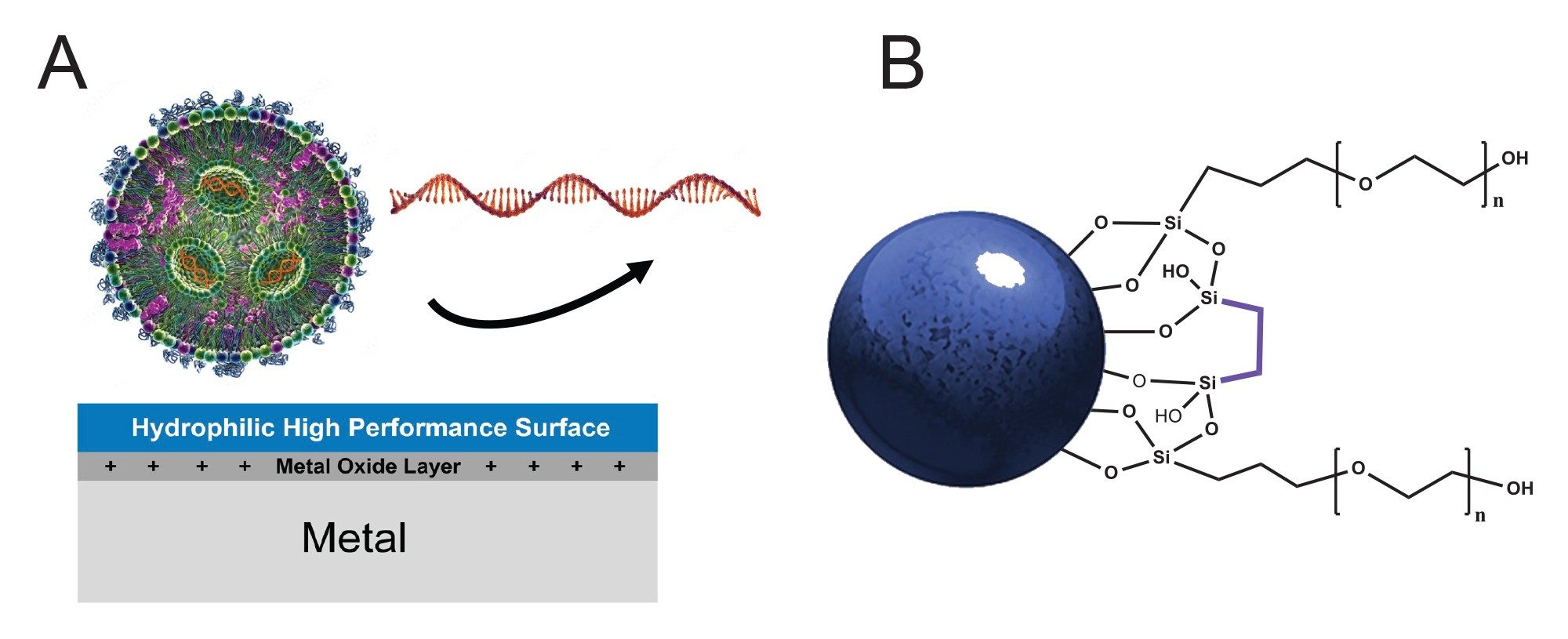
Figure 1 shows the design features of GTxResolve™ Premier SEC 1000 Å Column hardware surface and packing material bonding. During chromatography, biomolecules can interact with positively charged metal oxide layer in regular metallic column hardware leading to sample losses and inaccurate quantification. To prevent this, MaxPeak™ HPS High Performance Surfaces are to protect analytes from having an adsorptive interaction. This novel hardware is constructed with a hydrophilically modified organic/ inorganic hybrid layer, akin to a bridged ethylene hybrid particle composition. Reducing non-specific adsorption is critical for any impurity analysis or regulatory compliant because it will help ensure reproducible and accurate recoveries.
Another design and performance breakthrough for this new GTxResolve™ Premier SEC Column is its 3 μm high strength silica 1000 Å particle, which is a first-of-its-kind. The chemistry of this particle is modified with an inventive polyethylene oxide bonding and bridged ethylene hybrid crosslinks. This particle technology exhibits a new level of inertness combined with unique hydrophilicity and low ionic properties.
High Reproducibility Separations with Improved Resolution
High resolution and reproducible separation performance is critical for all analysts, whether they are working in an analytical development, process development, formulation, CMC, or release testing laboratory. This minimizes surprises during method development and validation and improves the overall confidence of the acquired data. With this in mind, GTxResolve™ Premier SEC 1000 Å 3 µm Columns were evaluated for their batch-to-batch and column-to-column reproducibility. Importantly, tests were performed with high molecular weight proteins and a ladder of increasingly larger nucleic acid analytes.
(1) Protein Analytes
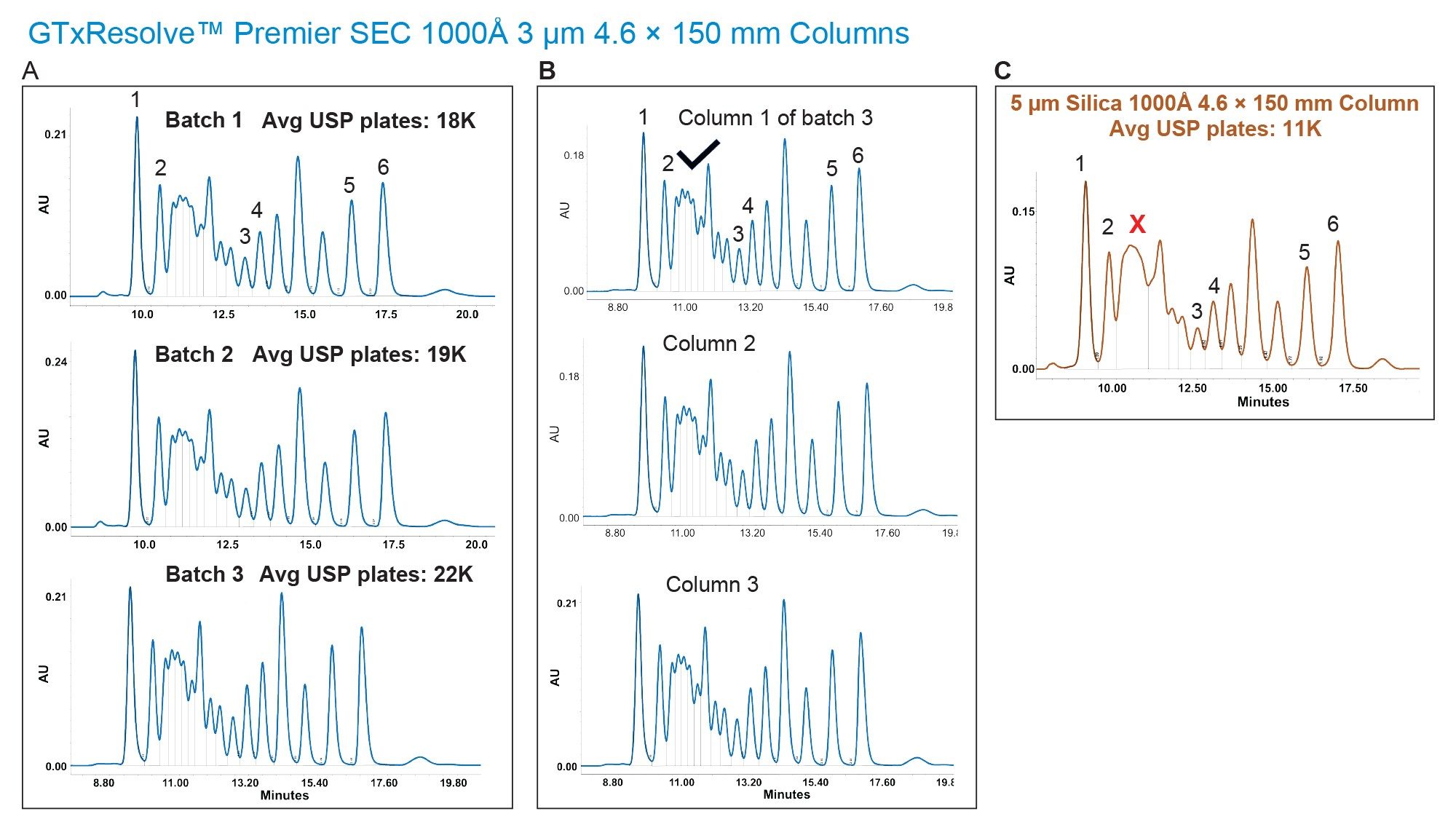
columns were first tested for SEC performance using a mixture of proteins, containing (in order of increasing size) myoglobin, bovine serum albumin, immunoglobulin, thyroglobulin monomer, and thyroglobulin dimer (Table 1). A representation of three batches and three columns for a set of 4.6 x 150 mm and 4.6 x 300 mm dimensions are shown in Figure 2. In this figure, it can also be seen that performance is compared against a commercially available 5 µm 1000 Å silica particle column. In general, the GTxResolve™ Premier SEC 1000 Å Columns exhibited higher signal (0.12 AU) in contrast to the 5 µm columns (0.09 AU). Furthermore, resolution between IgG and BSA peaks was greatly improved with the GTxResolve™ Premier SEC 1000 Å Columns. Superior performance of the GTxResolve™ Premier SEC 1000 Å Columns is also indicated by higher plate count. A 150 mm GTxResolve™ Premier SEC 1000 Å Column showed 19 K plates, an average for all peaks, versus the 9K obtained with the 5 µm silica comparison column. Doubling the flowrate (to 0.2 mL/min) for 300 mm column not only maintained higher plate count but even increased further for GTxResolve Premier SEC 1000 Å Column owing to the smaller particle size (3 µm). The resolution performance comparison of a 3 µm GTxResolve Premier SEC 1000 Å Column versus a 5 µm silica particle column is shown in Table 2.
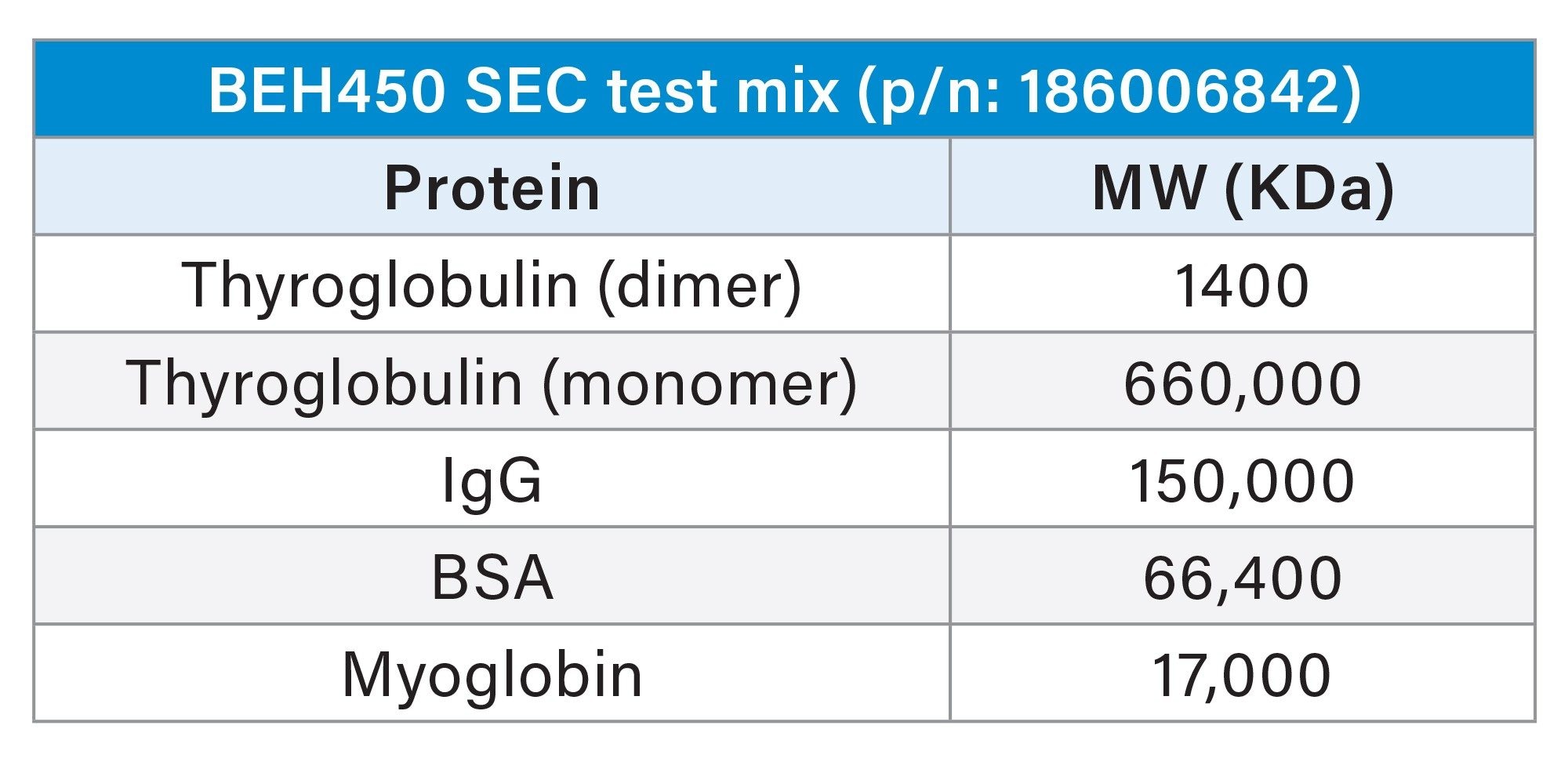

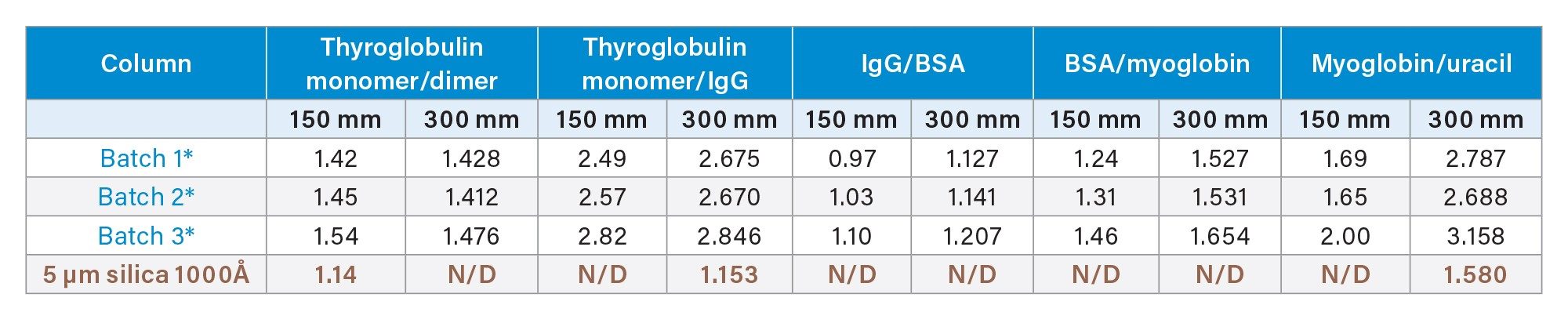
Table 2: Comparison of Resolution among protein mixture components.
*GTxResolve™ Premier SEC 1000 Å Column batches, N/D - no resolution detected.
(2) Nucleic acid analytes
Negatively charged nucleic acids are more vulnerable to non-specific adsorption to column surfaces compared to proteins. They are also very sensitive to the net charge of the packing material. In the past, it was unfortunately common for chromatographers to encounter batches of packing material that showed a difference in electrostatic properties and an ionic strength dependence that would mean differences in electrostatic repulsion and/or attraction of nucleic acid analytes. Therefore, it was critical that GTxResolve™ Premier SEC 1000 Å Columns be tested against a dsDNA 50 to 1350 ladder. Like with the protein separations, both batch to batch and column to column reproducibility was investigated. Like before, the chromatographic performance of a comparison 5 µm 1000 Å silica particle column was again assessed. As shown in Figure 3, consistent chromatographic profiles were obtained across all batches and all columns representing the GTxResolve™ Premier SEC 1000 Å 3 µm Column technology. Fine structure indicating 8 partially resolved species in the 766 to 400 bp elution window. Meanwhile, near baseline resolution was obtained for all other species, such as the 1350, 916, 350, 300, 100, and 50 bp species. This type of resolving power was not possible with the 5 µm silica particle comparison column. Separation efficiency is also indicated by USP plate averages of the resolved peaks. This value was 20 K for the GTxResolve™ Premier SEC 1000 Å Column and 11K for the 5 µm silica column. The enhanced resolution of the new column can be attributed to the optimized pore size distribution and the unique packing material of the GTxResolve™ Premier SEC 1000 Å Columns which allow for more effective separations of molecules based on their size differences. In short, the predicted amount of kinetic efficiency that should come from a 3 µm packing material is confirmed to be achieved.
Minimal Adsorption and Low Secondary Interactions
While not analytes intended for use with GTxResolve™ Premier SEC 1000 Å Column, monoclonal antibodies, such as NISTmAb, and antibody-drug conjugates, such as Kadcyla (ado-trastuzumab emtansine), are known to be highly susceptible to secondary interactions. Goyon, Fekete and co-workers proposed tests with these types of probe molecules back in 2017.4 The dependence of a column and these analytes on titrations of mobile phase additives has continued to be an insightful tool to guide SEC column development.3 Here, the approach is again applied, albeit to widepore SEC packing materials. The GTxResolve™ Premier SEC 1000 Å 3 µm Columns exhibited very minimal secondary interactions for both electrostatic and hydrophobic type adsorption events. This is based on the average % change in USP tailing (6% and 11% for electrostatic and hydrophobic interactions, respectively) for GTxResolve™ Premier SEC 1000 Å 3 µm Columns. In contrast, 5 µm silica columns exhibited 21% and 31% change in USP tailing for those respective secondary interactions (Table 3). Gaussian peaks were obtained no matter the additive levels and the extent of peak tailing change between low (0 mM) and high salt (200 mM) was seen to be minimal (6%). The addition of ACN in the mobile phase slightly altered Kadcyla (ado-trastuzumab emtansine) peak position, but the overall shape was well preserved for the GTxResolve™ Premier SEC 1000 Å Columns. In contrast, a 5 µm 1000 Å silica particle comparison column exhibited high peak tailing, broader peak width (1.5 minute) for Kadcyla (ado-trastuzumab emtansine) ADC while assaying for hydrophobic interactions. In contrast, GTxResolve™ Premier SEC 1000 Å 3 µm Column exhibited <0.4 minute peak width for the same analyte. Thus, the comparison column exhibited much higher changes in tailing factors upon the addition of mobile phase additive, most especially when acetonitrile was added to Kadcyla (ado-trastuzumab emtansine) separations.
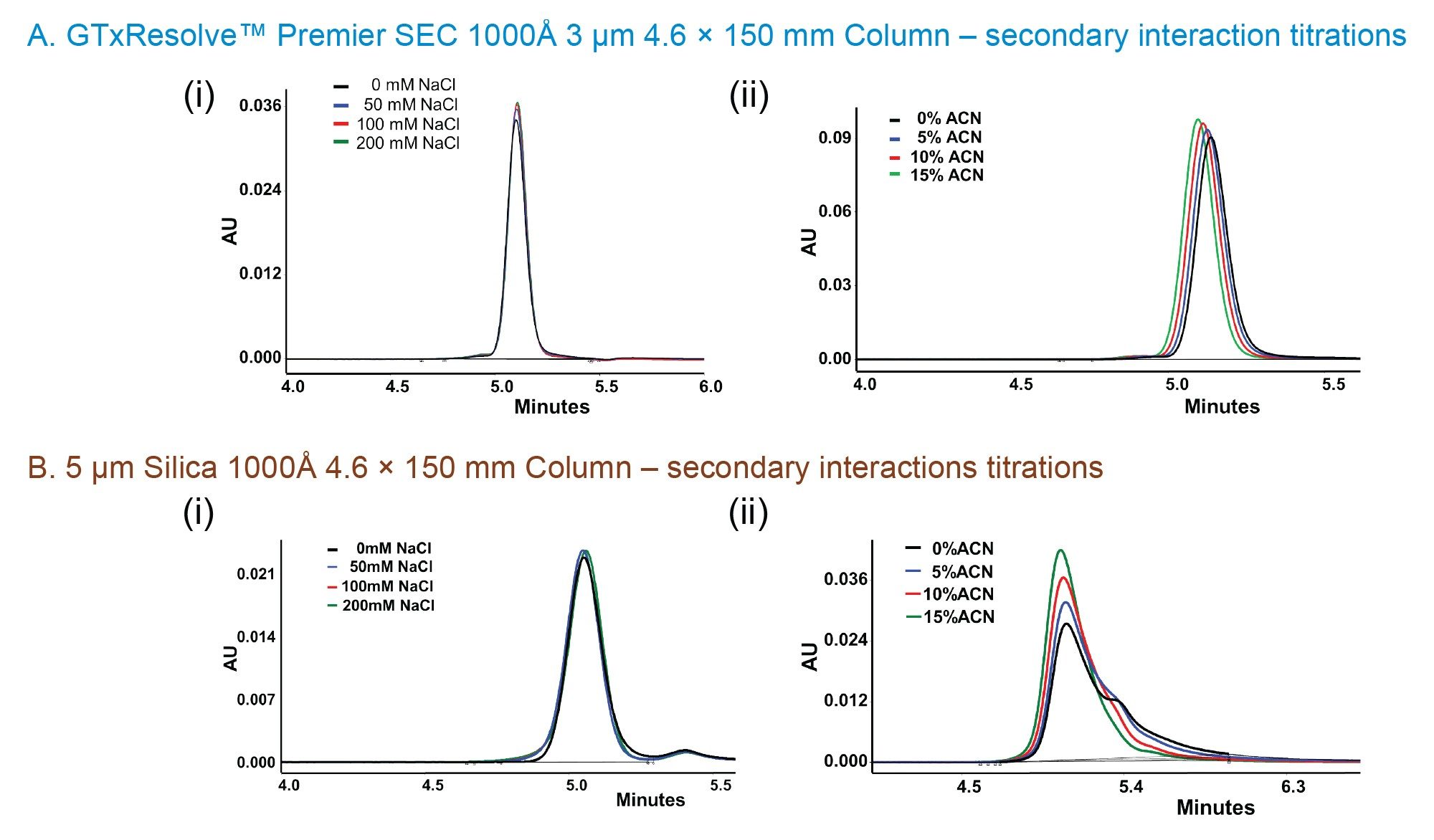
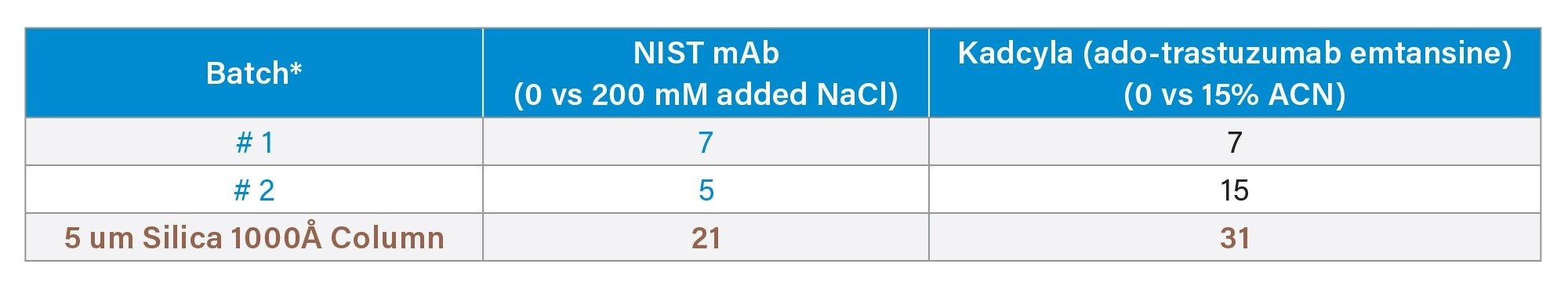
Table 3. Peak tailing percentage% changes in response to titrations against secondary interactions.
*GTxResolve™ Premier SEC 1000 Å Column batches
Conclusion
This application note highlights the advantages of GTxResolve™ Premier SEC 1000 Å 3 µm Columns and their ability to provide improved resolution and higher signal for biological macromolecules, including both proteins and nucleic acids. Its novel particles and MaxPeak™ HPS hardware minimize adsorptive sample losses while providing high separation efficiencies and reproducible chromatograms. These features provide a superior alternative to the currently available 5 µm silica 1000 Å SEC columns. Improvements of this sort will be critical to improving the characterizing new drug substances, such as mRNA, viral vectors, and lipid nanoparticles. The improved SEC performance provided by this GTxResolve™ Premier SEC 1000 Å Column will make it possible to document impurity profiles and elucidate important critical quality attributes on the way to preparing IND filings and finalize potency and safety indicating release tests.
GTxResolve, MaxPeak, ACQUITY and UPLC are trademarks of Waters Technologies Corporation. Kadcyla is a trademark of Genentech, Inc. All other trademarks are the property of their respective owners.
References
- H. G. Barth and B. E. Boyes, “Size Exclusion Chromatography,” Anal Chem, vol. 62, no. 12, pp. 268–303, Jun. 1990, doi: 10.1021/ac00211a020.
- P. Hong, S. Koza, and E. S. P. Bouvier, “A review size-exclusion chromatography for the analysis of protein biotherapeutics and their aggregates,” Journal of Liquid Chromatography and Related Technologies, vol. 35, no. 20. Taylor & Francis Group, pp. 2923–2950, Jan. 01, 2012. doi: 10.1080/10826076.2012.743724.
- S. Fekete, L. Kizekai, Y. T. Sarisozen, N. Lawrence, S. Shiner, and M. Lauber, “Investigating the secondary interactions of packing materials for size-exclusion chromatography of therapeutic proteins,” J Chromatogr A, vol. 1676, p. 463262, Aug. 2022, doi: 10.1016/J.CHROMA.2022.463262.
- A. Goyon, A. Beck, O. Colas, K. Sandra, D. Guillarme, and S. Fekete, “Evaluation of size exclusion chromatography columns packed with sub-3µm particles for the analysis of biopharmaceutical proteins.” J Chromatogr A, vol. 1498, pp. 80-89, May. 2017, doi:https://doi.org/10.1016/j.chroma.2016.11.056.
720008289, April 2024