
For forensic toxicology use only.
The principal goal of this work was to develop a single SPE-based analytical protocol suitable for determination of THC, OH-THC, and COOH-THC in either blood or urine samples and appropriate for either GC- or LC-based analysis. Other important goals were the demonstration of UPLC for rapid and efficient separation of the analytes for tandem electrospray MS/MS analysis and also the demonstration of tandem GC-MS/MS analysis as a complementary analytical procedure.
Δ9-Tetrahydrocannabinol (THC) is the principal psychoactive constituent of the cannabis products marijuana and hashish. After smoking or oral ingestion of cannabis, THC is incorporated into the bloodstream and is available for transport to receptor sites and for metabolism. Among the important THC metabolites are hydroxy-THC (OH THC), which is also psychoactive, and carboxy-THC (COOH-THC) which is not psychoactive but may have analgesic properties (see structures, Figure 1). THC and its metabolites may be detected in blood and urine. In blood, THC is detectable for many hours after ingestion and is indicative of recent cannabis ingestion. Therefore, such analysis is evidence that the user may have been under the influence of THC at the time the sample was collected. The THC level in the urine of users is generally very low; the principal analyte in urine is the COOH-THC metabolite. COOH-THC may be detectable in urine samples many hours or even days after ingestion of cannabis. Although the presence of the non-psychoactive metabolite is evidence that the subject has recently used cannabis, it is not necessarily evidence that the user was under the influence of THC at the time the sample was collected.
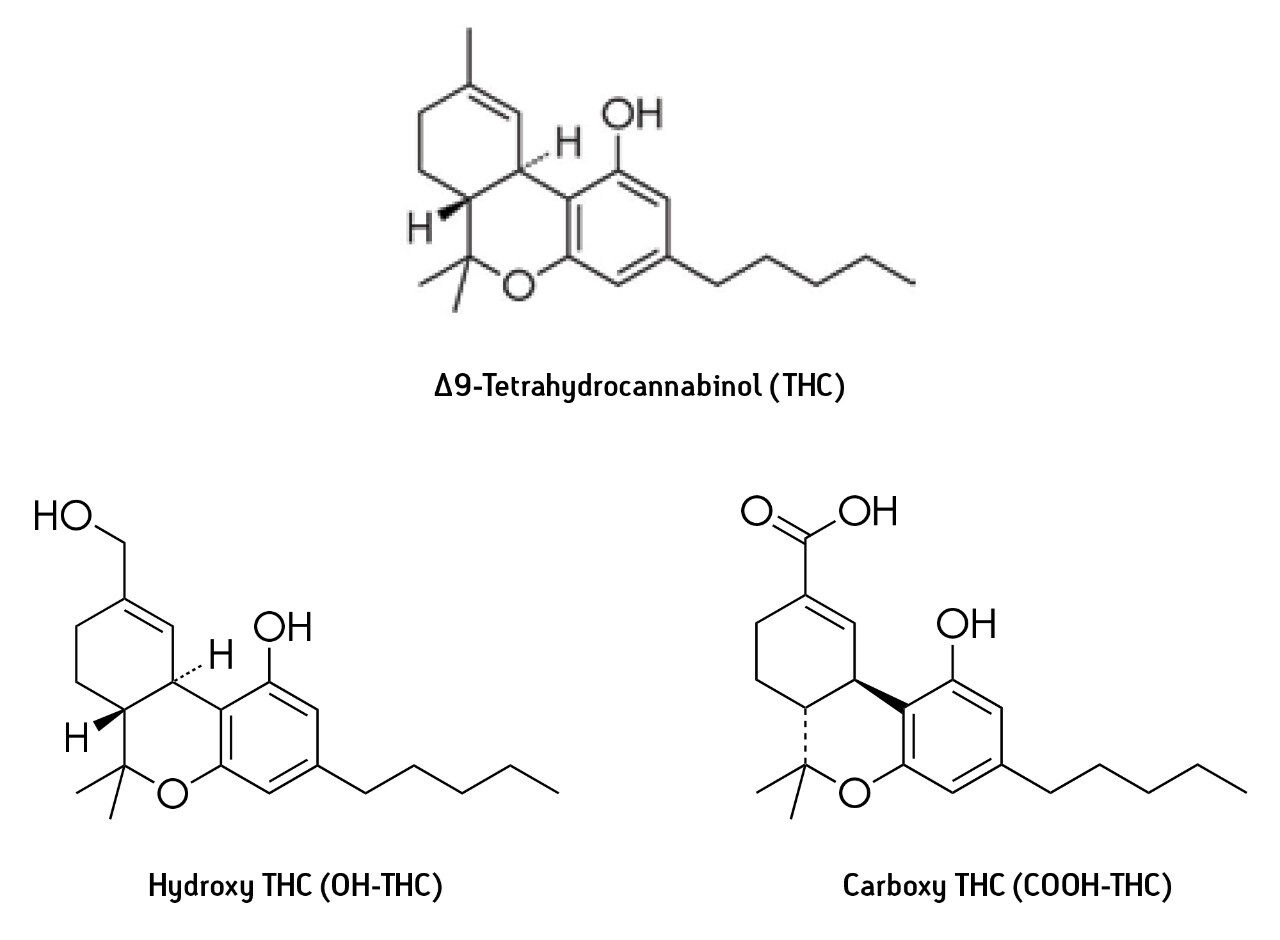
THC and metabolites are commonly determined in blood and urine using GC-MS or LC-MS techniques after sample preparation using solid-phase extraction (SPE). Blood is usually precipitated with acetonitrile or other organic solvent prior to SPE. Urine is typically treated with strong base prior to SPE to hydrolyze conjugated forms of the metabolites such as COOH-THC-glucuronide. GC-MS analysis is performed after silylation or other appropriate derivatization. LC-MS analysis is commonly performed using electrospray ionization in positive mode (ESI+) for THC and negative mode (ESI-) for COOH-THC. OH-THC can be detected using positive or negative electrospray; positive mode was used for this study.
The principal goal of this work was to develop a single SPE-based analytical protocol suitable for determination of THC, OH-THC, and COOH-THC in either blood or urine samples and appropriate for either GC- or LC-based analysis. Other important goals were the demonstration of UPLC for rapid and efficient separation of the analytes for tandem electrospray MS/MS analysis and also the demonstration of tandem GC-MS/MS analysis as a complementary analytical procedure.
Silica-based SPE cartridges are available which provide good performance, but separate methods are required for urine and blood samples. Also, precipitated blood samples must be evaporated and reconstituted in aqueous buffer prior to SPE. Since THC is highly water insoluble, losses of this analyte may occur during this reconstitution step. Using the Oasis MAX SPE protocol discussed in this application note, the precipitated blood sample need not be evaporated. Instead, a simple modest dilution step is performed prior to SPE loading. Oasis MAX is a mixed-mode strong anionexchange sorbent. It demonstrates excellent reversed-phase retention of neutrals or weak acids such as THC, and mixed-mode retention of acids such as COOH-THC. The 3 cc Oasis MAX cartridge discussed in this application note gives superior performance compared with 6 cc silica-based cartridges. Analyte recovery and sample cleanliness are equivalent or better and sample pre-preparation for blood samples is simpler and quicker because no evaporation step is required prior to SPE.
For both blood and urine, three calibration curves were prepared and analyzed in a single day for intra-day method evaluation and three calibration curves were prepared (one per day) over a three day period for inter-day method evaluation. Consistent performance was demonstrated by comparison of curves prepared in urine obtained from three separate donors.
Matrix effects (ion suppression or enhancement) were evaluated by comparison of absolute response observed in blank samples spiked after SPE (5 replicates) to response seen in standards prepared in mobile phase. Recovery was evaluated from response observed in spiked samples with internal standard added before SPE, compared to response observed in spiked samples with internal standard added after SPE.
Standard compounds for this study were obtained from Cerilliant (Round Rock, TX). Internal standards used were THC-d3, OH-THC-d3 and COOH-THC-d3.
Stabilized blood was obtained from Lampire Biologicals (Pipersville, PA).
Urine samples were obtained from donors at Waters Corporation.
Sylon BFT (BSTFA/TMCS 99:1) was obtained from Sigma-Aldrich (St. Louis, MO).
Oasis MAX, 60 mg (pn: 186000367) or flangeless (pn 186001884) were from Waters Corporation (Milford, MA).
Calibration solutions were added to produce calibrators (0.5 mL) covering the range from 0 to 100 ng/mL. Internal standards were added to the blank samples and calibrators at a concentration of 50 ng/mL. All samples were then precipitated by dropwise addition of 1 mL acetonitrile while vortex mixing. After centrifugation, 1 mL of the supernatant was diluted to 2.5 mL with 1 % aqueous ammonia. The resulting solution was loaded onto the SPE cartridge.
Calibration solutions were added to produce calibrators (2.0 mL) covering the range from 0 to 100 ng/mL for COOH-THC and from 0 to 50 ng/mL for OH-THC and THC. Internal standards were added to the blank samples and calibrators at a concentration of 50 ng/mL. The samples were then hydrolyzed by addition of 50 μL of 10 N NaOH solution followed by heating at 60 °C for 15 minutes. After cooling, the samples were adjusted to pH 7 by addition of 50 μL of 50% aqueous acetic acid and 200 μL of 0.1 M pH 7 phosphate buffer. 1 mL of acetonitrile was added to the prepared sample and the resulting solution was loaded onto the SPE cartridge.
The same SPE protocol (Figure 2) was employed for all samples, blood or urine. Only the reconstitution and derivatization steps differ for GC-MS or LC-MS analysis.
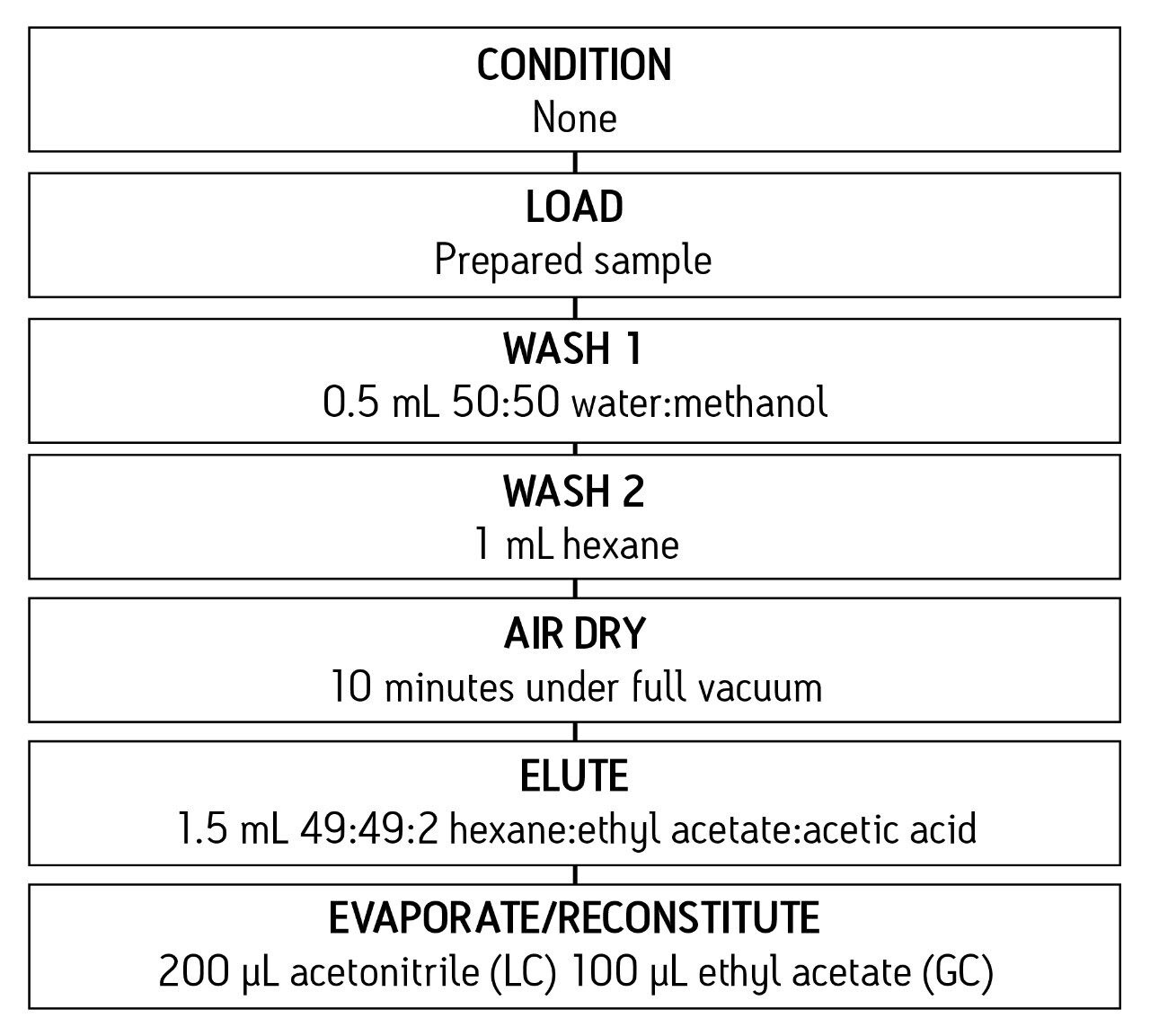
The SPE cartridges were eluted into Teflon lined screw-cap vials. The SPE eluent was evaporated and reconstituted in 100 μL of ethyl acetate. 50 μL of derivatization reagent was added and the capped vial was placed in a 70 °C oven for 15 minutes. After cooling, the derivatized sample was transferred to an autosampler vial and analyzed by GC-MS/MS.
The SPE eluent was evaporated and reconstituted in 200 μL of acetonitrile. After vortexing, 150 μL of water was added the sample was mixed well and then analyzed by LC-MS/MS.
The SPE based analytical procedure was demonstrated using tandem UPLC-MS and Tandem GC-MS. The instruments used are shown in Figure 3.

Chromatographic conditions and MRM transitions are presented in Tables 1 and 2. Typical LC-MS and GC-MS chromatograms are presented in Figures 4 and 5.
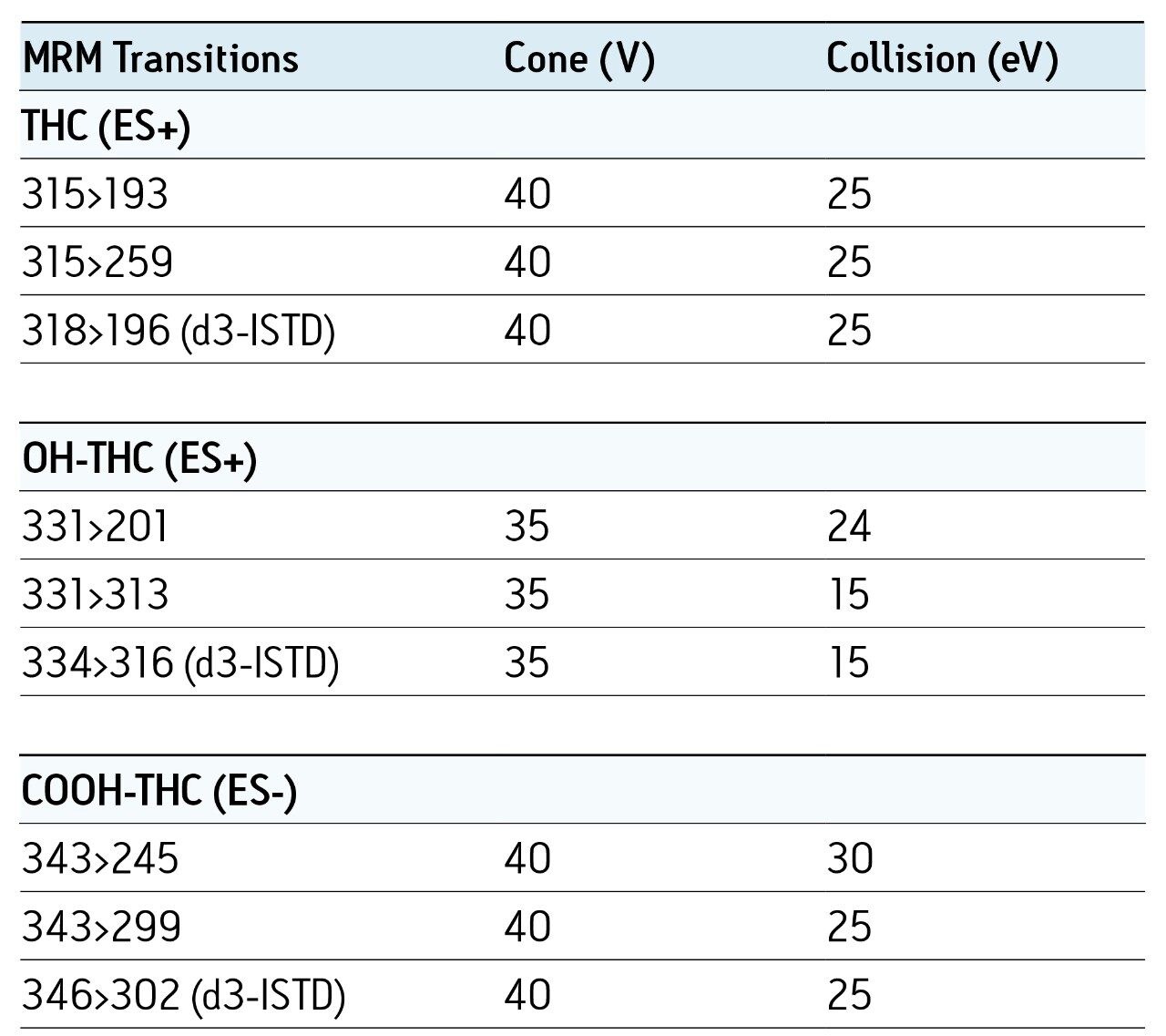
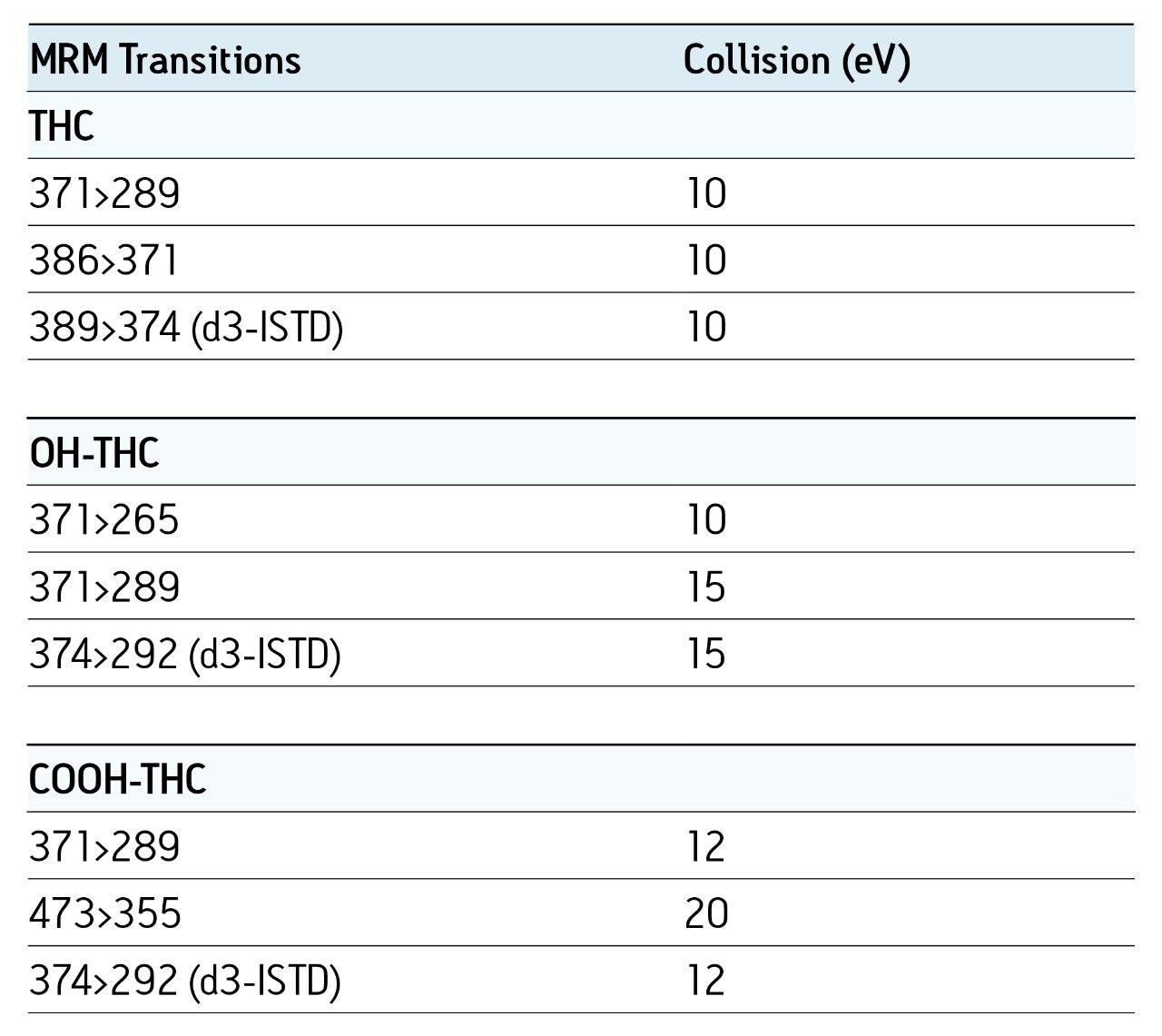
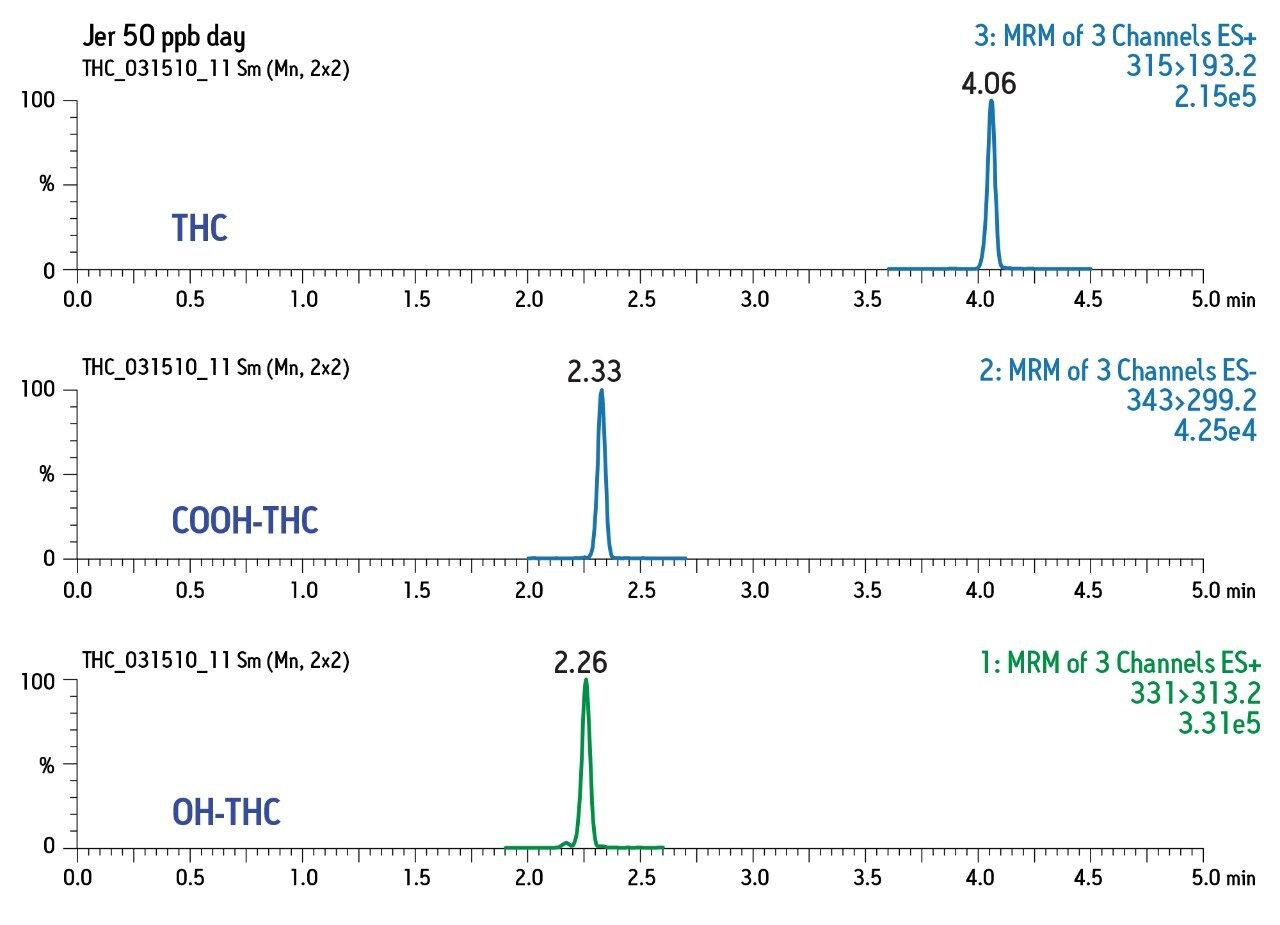

Consistent, reproducible, linear results were obtained for both matrices and for both GC- and LC-based analysis.
Intraday calibration (3 curves, n = 18) gave correlation (r2) of 0.997 and interday correlation (3 curves, n = 18) of 0.993 for COOH-THC. Similar results were obtained for OH-THC and THC (3 curves, n = 15). Figure 6 shows the interday results for COOHTHC determined in urine obtained over the period of three days.
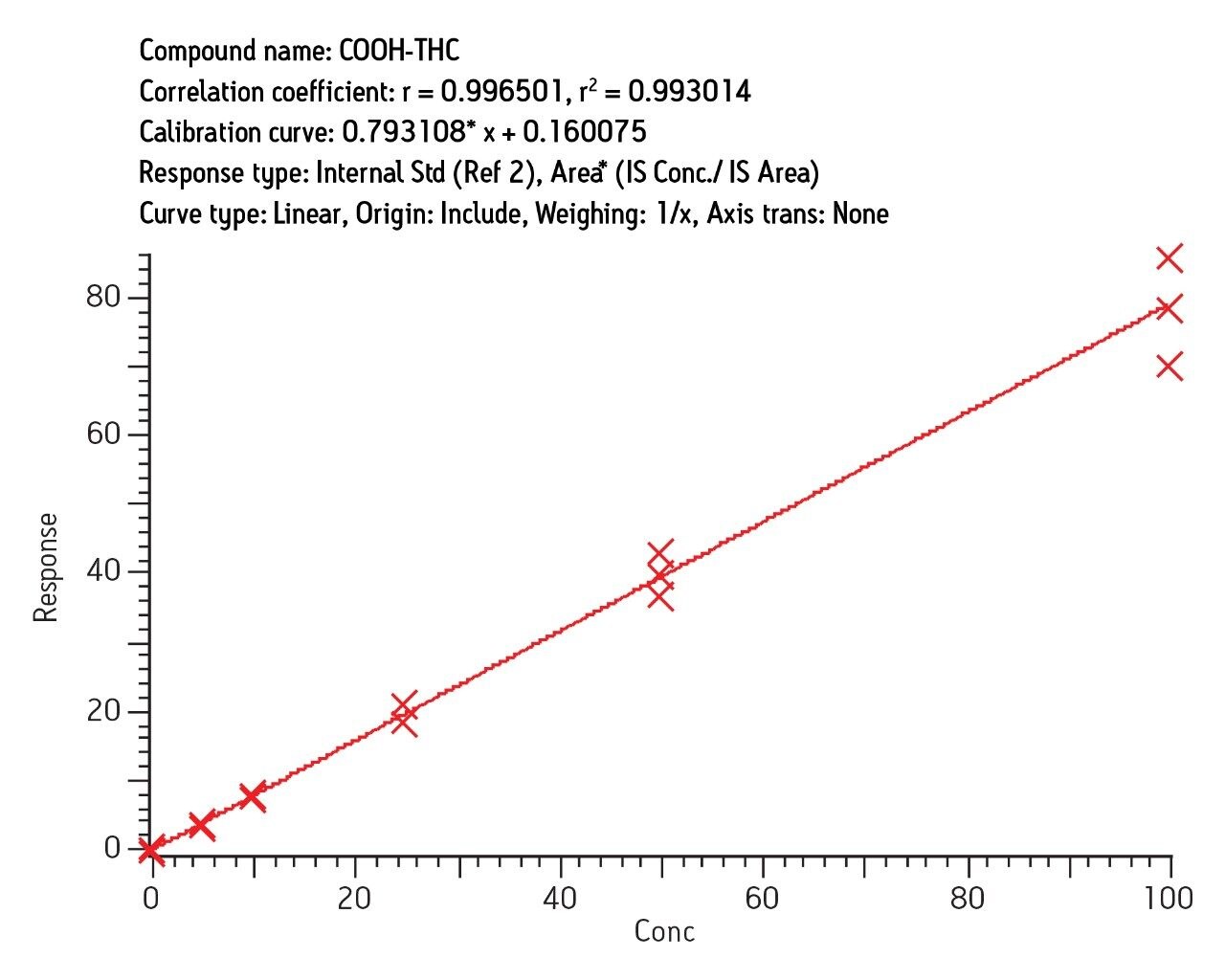
Recovery was 92% (7% RSD) and ion suppression was under 10% for COOH-THC. Recovery and reproducibility was similar for other analytes. Replicate analysis of 0.5 ng/mL spiked samples showed RSD of 6–10% at that level for all analytes. A very conservative estimate of LOQ is therefore 0.5 ng/mL.
Intraday calibration (3 curves, n = 18) gave correlation (r2) of 0.996 and interday correlation (3 curves, n = 18) of 0.990 for THC. Similar results were obtained for OH-THC and COOH-THC (3 curves, n = 18). Figure 7 shows the intraday results for THC determined in urine, three independent curves run on the same day.
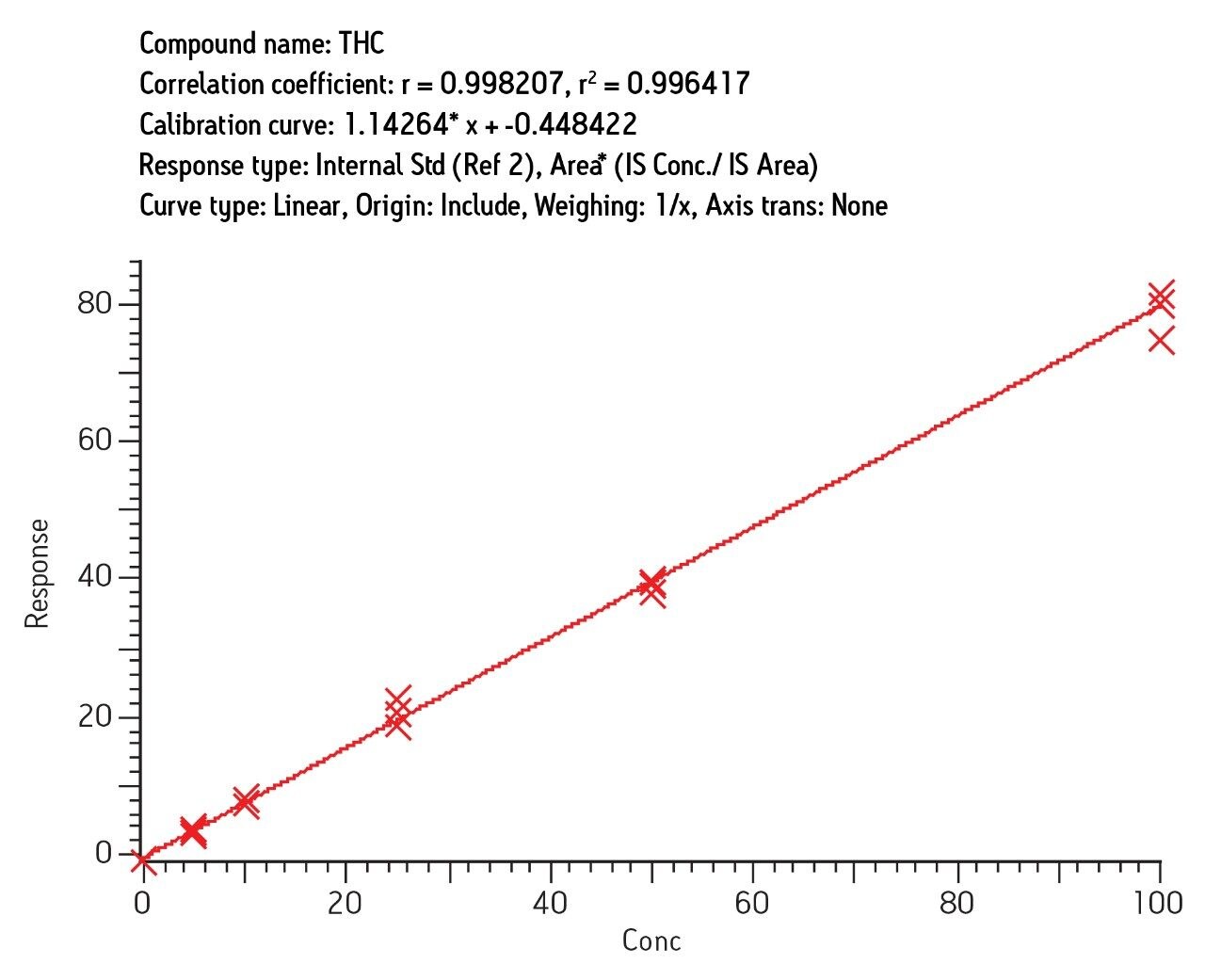
Recovery was 78% (7% RSD) and ion-suppression was under 10% for THC. Recovery and reproducibility was similar for other analytes. However, much of the recovery loss results from the initial acetonitrile precipitation step. The actual SPE recovery is similar to that observed for urine (c.a. 90%). Replicate analysis of 0.5 ng/mL spiked samples showed RSD of 8–14% at that level for all analytes. A very conservative estimate of LOQ is, therefore, 0.5 ng/mL.
For both urine and blood, excellent results were obtained similar to results reported for the LC/MS portion of this study. Figure 8 shows a typical calibration curve for COOH-THC in urine. Equivalent performance was obtained for the other analytes in urine and blood.
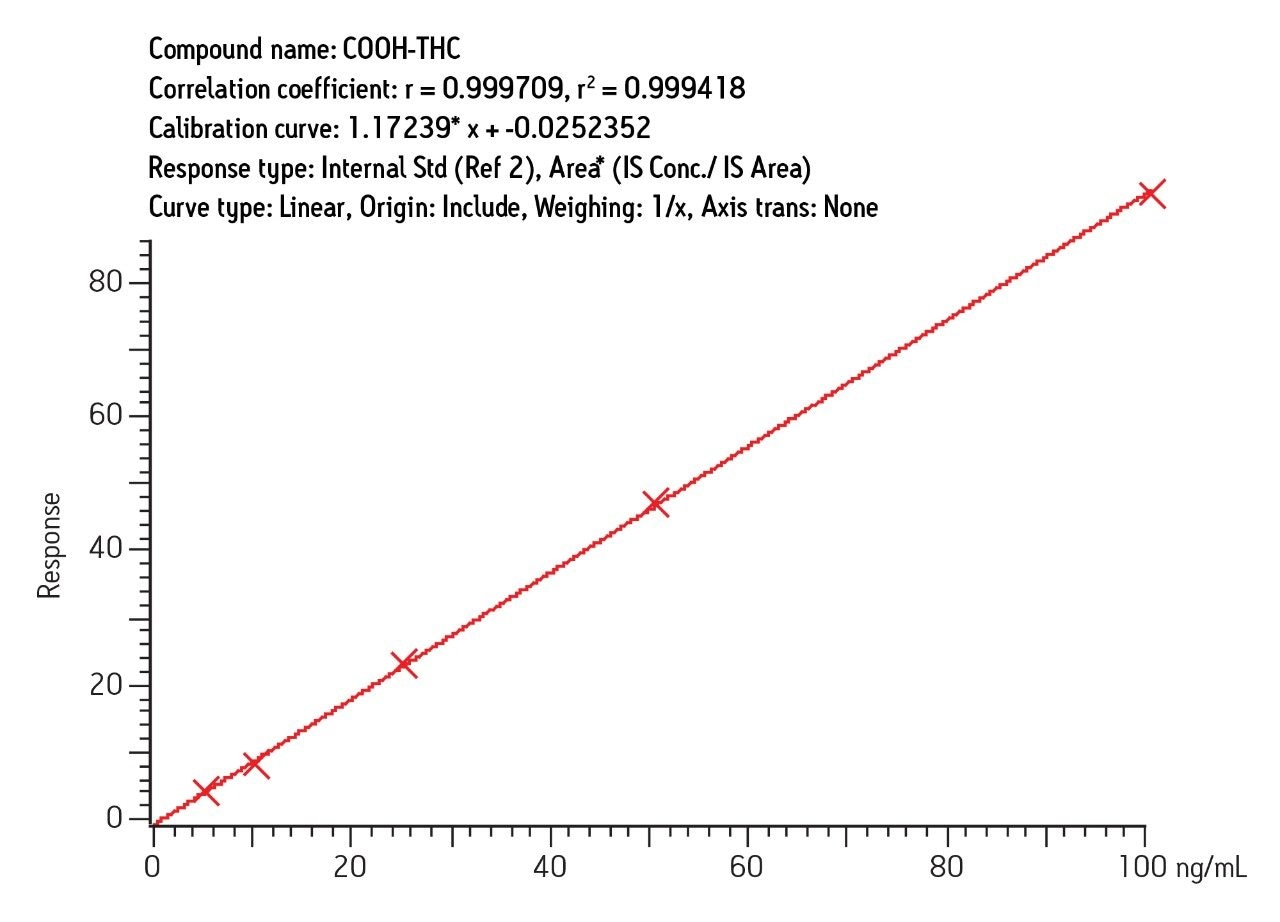
The results demonstrate the value of a single SPE protocol for preparation of blood or urine samples for either LC-MS or GC-MS analysis. The reason that the SPE protocol is so effective for this analysis is the nature of the Oasis MAX sorbent, a mixed-mode anion exchanger with outstanding reversed-phase performance. Unlike silica-based mixed-mode sorbents, Oasis MAX sorbent shows excellent retention of THC and its major metabolites even when the sample load is as high as 40% acetonitrile. For blood samples, this fact is very important because the blood is precipitated with acetonitrile. Other protocols would require evaporation and reconstitution of the precipitated blood prior to SPE. Moreover, THC and OH-THC are highly insoluble in the aqueous buffers used for such reconstitution. With Oasis MAX cartridge, no such elaborate prepreparation step is required. The precipitated blood is simply diluted to 40% or lower acetonitrile content prior to SPE.
The results demonstrate the suitability of the SPE protocol for either LC-MS/MS or GC-MS/MS determination of THC and its primary metabolites in blood or urine. Detection limits, accuracy and precision are similar for both types of analysis. Of course, the GC-based analysis requires a derivatization step that is not needed for LC analysis. For selected ion recording using single quad GC-MS (SIR), results are also equivalent or superior to results obtained using silica based mixed-mode SPE. During this study, hundreds of urine and blood samples were analyzed by GC-MS and LC-MS. No injector, column or mass-spectrometer maintenance was required during the course of the study.
Thanks to Adam Ladak, Waters Manchester, for development of the GC-MS derivatization strategy.
720003738, September 2010