
In this study, we will examine the improved carryover obtained after design optimization of the seal pack components within the Alliance HPLC System injector assembly.
Improved carryover performance for the 2018 Alliance HPLC System with the newly designed injector assembly
Sample carryover is a common problem for analytical laboratories and may adversely affect chromatographic methods. Sample carryover occurs when material from an injection is present in subsequent injections. The impact of carryover may result in failing batches, out of specification results, and poor reproducibility, to name a few. There are several factors that can influence carryover including the chemistry of the analyte, the analytical column, and the design of the HPLC system injector assembly. For example, an HPLC system based upon a flow-through needle design can provide reduced carryover because the interior of the sample injection needle is continuously washed by the mobile phase. In addition, most LC systems include some type of needle washing mechanism to rinse the exterior of the needle with an appropriate wash solvent in order to further reduce sample carryover.
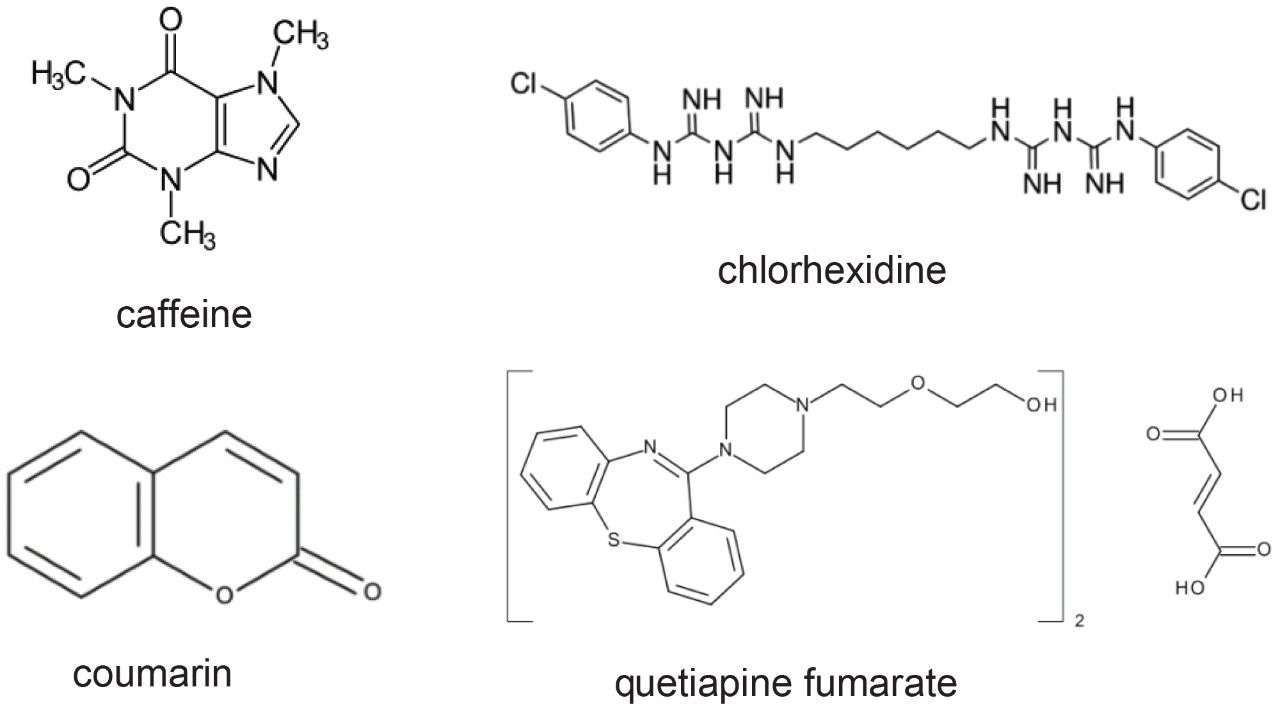
In this study, we will examine the improved carryover obtained after design optimization of the seal pack components within the Alliance HPLC System injector assembly. Four compounds, each with different chemical properties, were selected to demonstrate the injector design improvements.
|
LC systems |
|
|---|---|
|
Alliance: |
Alliance e2695 Separations Module with 100 μL syringe, 2998 PDA Detector, and CH-30 equipped with passive column preheater. Firmware 3.03 |
|
2018 Alliance: |
2018 Alliance: Alliance e2695 Separations Module with 100 μL syringe, 2998 PDA Detector, CH-30 equipped with passive column preheater and the e2695 Enhancement. Firmware 3.04 |
|
Caffeine: |
Challenge solution: 4.0 mg/mL Caffeine (Solution 7), Standard at 0.01%: 0.4 μg/mL Caffeine (Solution 8), and Blank (Solution 9) from Waters HPLC with UV Standards Kit (p/n: 700003741) |
|
Column: |
XBridge BEH C18, 3.5 μm, 4.6 × 50 mm (p/n: 186003031) |
|
Column temp.: |
35 °C |
|
Sample temp.: |
4 °C |
|
Injection volume: |
10 μL |
|
Flow rate: |
1.0 mL/min |
|
Needle wash: |
Methanol |
|
Needle wash time: |
Normal |
|
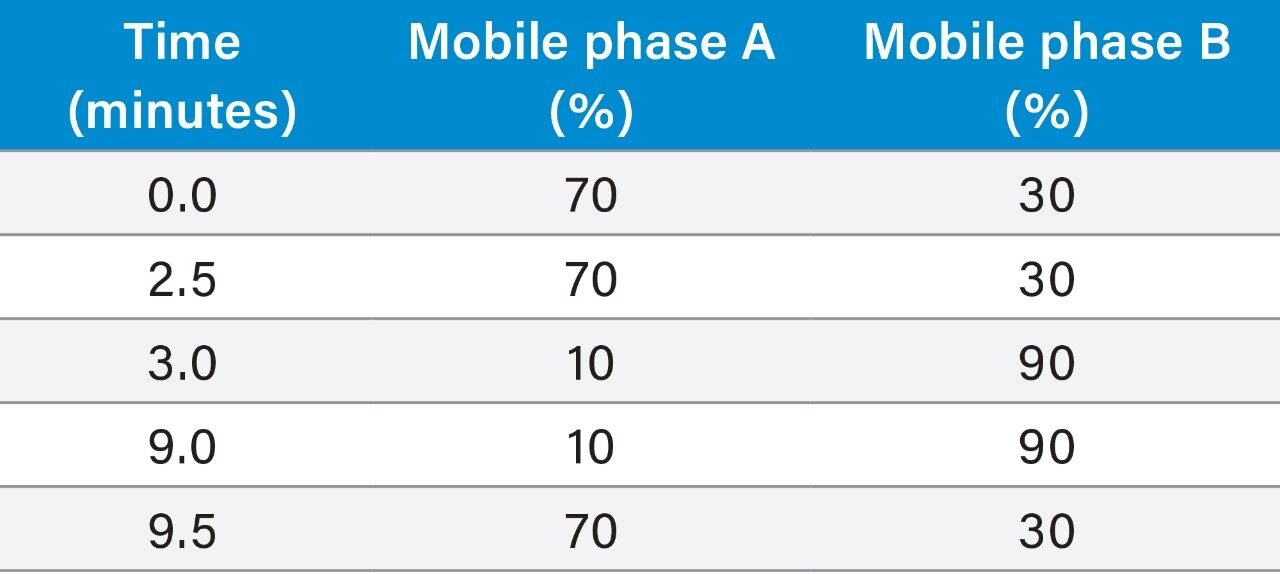
Mobile phase A: |
Water |
|
Mobile phase B: |
Methanol |
|
Run time: |
23 minutes |
|
PDA wavelength: |
273 nm at 1.2 nm resolution |
|
Chlorhexidine: |
Standard Solution: 1.0 mg/mL chlorhexidine in 0.1% TFA in water. Blank: 90:10 water:acetonitrile |
|
Column: |
CORTECS C18, 2.7 μm, 3 mm × 100 mm (p/n: 186007372) |
|
Column temp.: |
50 °C |
|
Sample temp.: |
Room temp. |
|
Injection volume: |
5 μL |
|
Flow rate: |
1.0 mL/min |
|
Needle wash: |
50:50 water:acetonitrile |
|
Needle wash time: |
Normal |
|
Mobile phase A: |
0.1% TFA in water |
|
Mobile phase B: |
0.1% TFA in acetonitrile |
|
Gradient: |
Isocratic (67:33 mobile phase A: mobile phase B) |
|
Run time: |
10 minutes |
|
PDA wavelength: |
257 nm at 4.8 nm resolution |
|
Coumarin: |
Stock solution: 8 mg/mL coumarin in methanol. Challenge solution: 2 mg/mL coumarin in water. Standard at 0.01%: 0.2 μg/mL coumarin in water. Blank: water |
|
Column: |
CORTECS C18 , 2.7 μm, 3 mm × 100 mm (p/n: 186007372) |
|
Column temp.: |
30 °C |
|
Sample temp.: |
4 °C |
|
Injection volume: |
4 μL |
|
Flow rate: |
0.8 mL/min |
|
Needle wash: |
90:10 water:acetonitrile |
|
Needle wash time: |
Normal |
|
Mobile phase A: |
Water |
|
Mobile phase B: |
Acetonitrile |
|
Gradient: |
Isocratic (90:10 mobile phase A: mobile phase B) |
|
Run time: |
15 minutes |
|
PDA wavelength: |
275 nm at 4.8 nm resolution |
|
Quetiapine Fumarate Assay USP 40 NF35 S11 |
|
|---|---|
|
Quetiapine: |
Standard solution: 0.16 mg/mL of quetiapine fumarate in mobile phase (standard stock solution for USP monograph assay). Blank: water |
|
Column: |
XBridge BEH C8, 5 μm, 4.6 mm × 250 mm (p/n: 186003018) |
|
Column temp.: |
25 °C |
|
Sample temp.: |
4 °C |
|
Injection volume: |
50 μL |
|
Flow rate: |
1.3 mL/min |
|
Needle wash: |
90:10 water:acetonitrile |
|
Needle wash time: |
Normal |
|
Mobile phase: |
54:7:39 methanol: acetonitrile: buffer premixed and filtered with 0.45 μm filter |
|
Buffer: |
2.6 g/L of dibasic ammonium phosphate adjusted to pH 6.5 with phosphoric acid |
|
Gradient: |
Isocratic |
|
Run time: |
15 minutes |
|
PDA wavelength: |
230 nm at 4.8 nm resolution |
Empower 3 Chromatography Data Software, FR 3, Hot Fix 1
In order to examine the impact of the optimized injector design on sample carryover, multiple compounds with varying chemical properties were analyzed on the Alliance HPLC System, and the 2018 Alliance HPLC System equipped with the e2695 Enhancement Kit. The compounds include caffeine, chlorhexidine, coumarin, and quetiapine fumarate. Each compound was prepared individually and injected in replicates of six.
In this study, two methods were used to assess carryover. One method uses a highly concentrated challenge solution, which saturates the detector. Because an accurate peak area cannot be defined due to detector saturation, a standard solution prepared at 0.01% of the challenge solution concentration is also injected. The injection sequence is as follows: a pre-blank, standard solution, challenge solution, post-challenge blank.
Carryover is then calculated by:
% carryover = (post-challenge blank peak area)/(standard peak area) * 0.01
This methodology was used to assess carryover for caffeine and coumarin. In the second methodology, the challenge solution falls within the linear range of the detector, allowing for the quantification of carryover based on the challenge sample solution. For this method, carryover is calculated by:
% carryover = (post-challenge blank peak area)/(standard peak area) * 100
This methodology was used for chlorhexidine and quetiapine fumarate.
The injector of the Alliance HPLC System uses a flow-through needle, which provides washing of the interior of the needle during the programmed method. The exterior surface of the needle is washed with an additional wash solvent for a specified time while the needle is seated in the seal pack of the injector assembly.2 The 2018 Alliance HPLC System includes a re-designed seal pack that improves and optimizes the needle wash flow over the exterior surface of the injector needle. For existing Alliance systems, an e2695 Enhancement Kit is available to convert the injector components to the new seal pack design.
To illustrate the impact of these changes, carryover of four analytes was evaluated on the Alliance HPLC System, and the 2018 Alliance HPLC System (Table 1 and Figure 2). Two common compounds that are regularly used to assess potential carryover on an HPLC system are caffeine and chlorhexidine, while coumarin and quetiapine fumarate are compounds known to carryover on HPLC systems.3,4
Both Alliance HPLC systems showed low levels of carryover for all compounds evaluated under the specified conditions. The 2018 Alliance HPLC System, however, was able to significantly reduce carryover further due to the improvements in the seal pack design. Specifically, caffeine carryover was reduced by a factor of seven, chlorhexidine carryover was reduced by a factor of twenty, coumarin carryover was reduced by a factor of three, and quetiapine carryover was reduced by a factor of one point five.
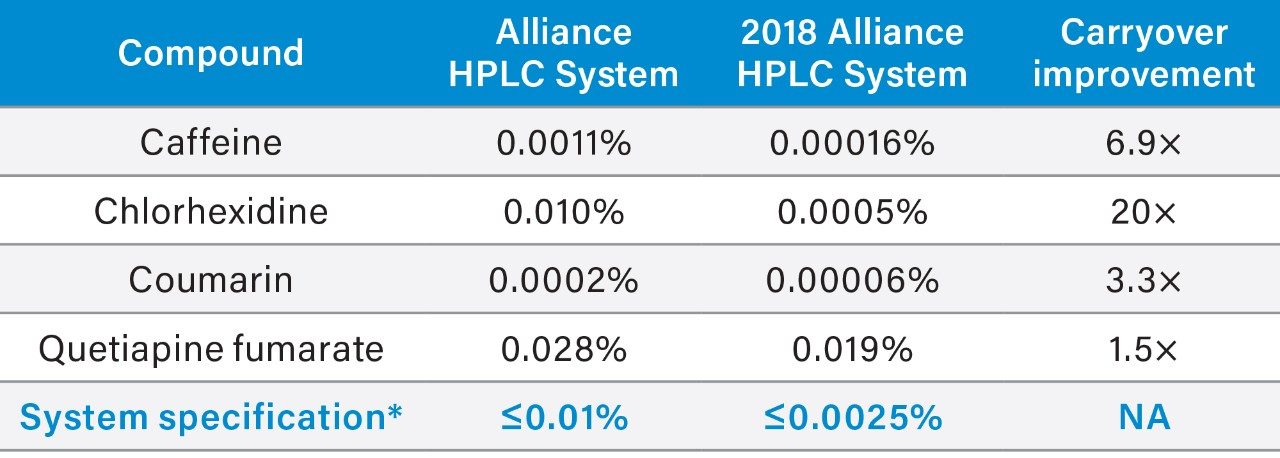
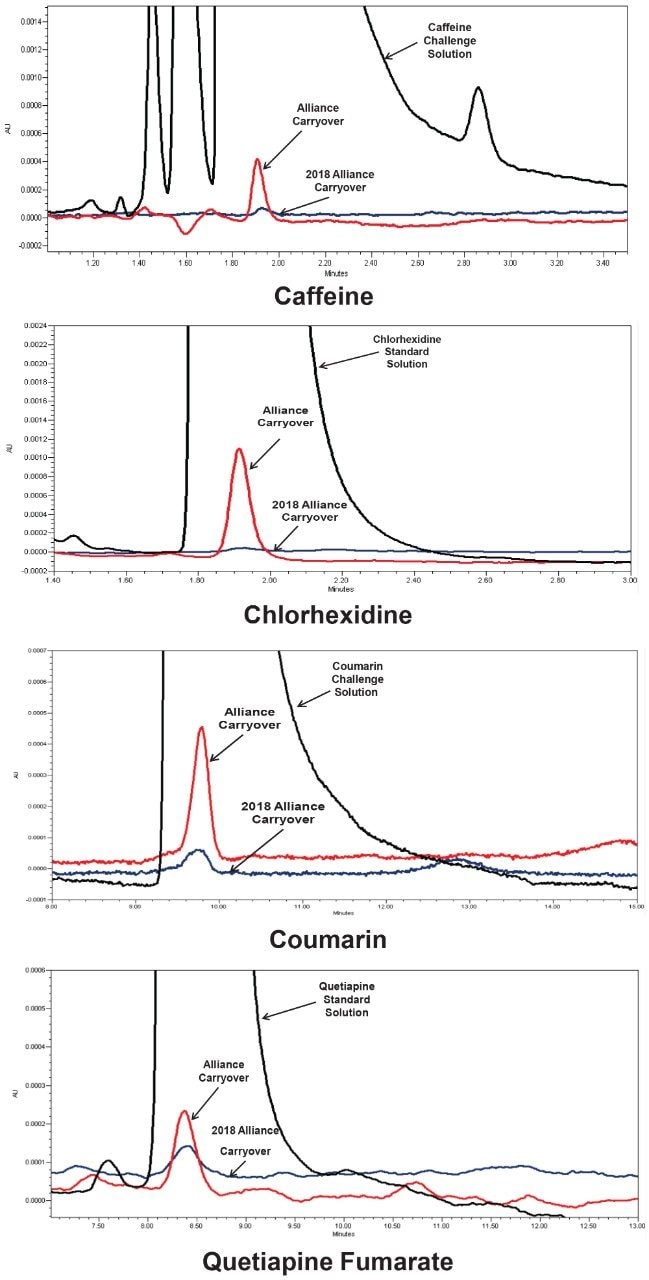
The 2018 Alliance HPLC System incorporates a newly designed seal pack which improves needle wash flow over the exterior of the injector needle. This newly designed seal pack results in a significant reduction in carryover for a wide range of chemical compounds. This data suggests that the 2018 Alliance HPLC System can reduce the amount of carryover present regardless of the specific compounds being analyzed.
720006386, October 2018