For research use only. Not for use in diagnostic procedures.
A rapid, targeted UPLC-MS/MS methodology has been developed for the semi-quantitative analysis of 57 diacyl-phosphatidylcholine species in human serum. This research method has demonstrated to be suitable for the analysis of these analytes at physiologically relevant levels in human serum. This LipidQuan-R method is optimized on the same versatile LC-MS platform that has previously been utilized for the analysis of various classes of compounds including bile acids, amino acids, free fatty acids, tryptic peptides, acylcarnitines, tryptophan metabolites, and various lipids. This new method extends the LipidQuan-R/MetaboQuan-R suite of methodologies, and allows the analysis of an expanding set of metabolites and lipids, when operated sequentially in a targeted multi-omics workflow.
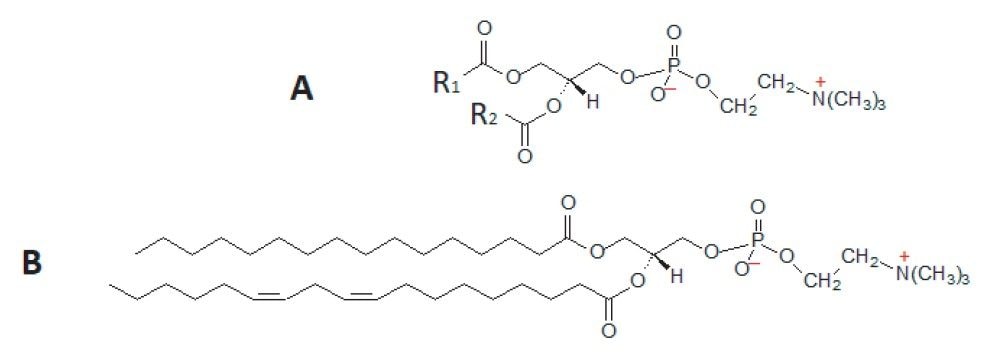
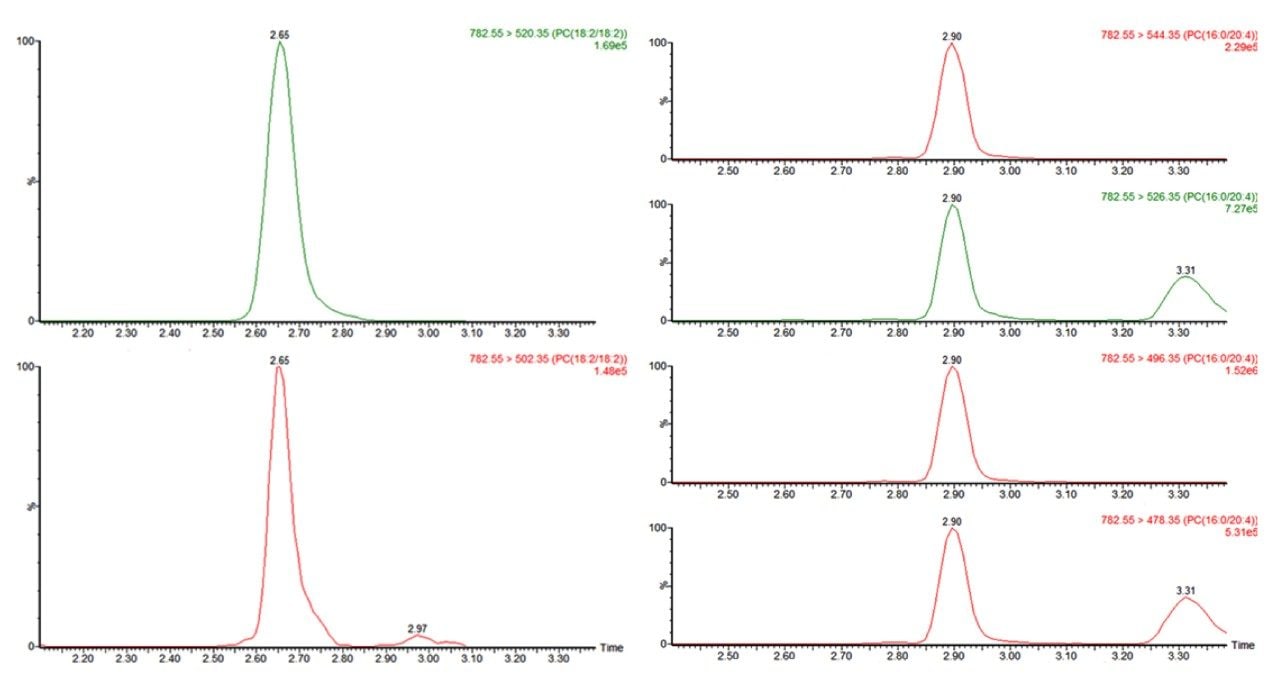
Diacyl phosphatidylcholines (diacyl-PCs) are lipid molecules made up of 2 fatty acids connected, via ester linkages, to a glycero-phosphocholine head group (i.e. 1,2-diacyl-sn-glycero-3-phosphocholines). See Figure 1 for a generic structure and a specific example. They are highly abundant lipids in human systems and are one of the predominant lipids that make up all cell membranes. Other forms of PCs exist in biological systems but most PCs exist in the diacyl form. This class of compound has huge variety in terms of the fatty acids that can be attached to the glycero-phosphocholine head group. Here, a UPLC-MS/MS method is presented that specifically and semi-quantitatively measures 57 individual diacyl-phosphatidylcholine species. Historically, this type of compound would have been detected using tandem quadrupole mass spectrometry using the m/z=184 product ion, as this gives the greatest response. This transition, however, lacks specificity and is prone to giving significant interference for certain analytes. The current method uses a quartet of MRM transitions to specifically target given diacyl-PC species. These 4 MRM transitions result from fragmentation patterns that involve the loss of the fatty acid residues from the molecule. Therefore, they make it possible to be very specific about the lipids being detected and in some cases can even distinguish between structural isomers. For example, PC 36:4 (i.e. a diacyl-PC where the 2 fatty acids are made up of 36 carbon atoms and 4 double bonds) could be either of the most two most common isomers PC(18:2_18:2) or PC(16:0_20:4). The current method can distinguish between and measure the lipids esterified in each PC species, thereby enabling a high degree of specificity and identification of individual PC species.
Human serum was protein precipitated with propan-2-ol at a ratio of 4:1 propan-2-ol:serum. This was then centrifuged for three minutes at 25,000 g. The resulting supernatant was then diluted 1:1 using deionized water and mixed. 5 μL was then injected onto the UPLC-MS/MS system.
UPLC separation was performed with an ACQUITY UPLC I-Class System (Fixed Loop), equipped with a CORTECS T3 2.7 μm (2.1 x 30 mm) Column. A sample of 5 μL was injected at a flow rate of 0.40 mL/min. Mobile phase A was 0.01% formic acid (aq) containing 0.2 mM ammonium formate and mobile phase B was 50% isopropanol in acetonitrile containing 0.01% formic acid and 0.2 mM ammonium formate. After an initial 1 minute hold at 70% mobile phase B, the phosphatidylcholines were eluted from the column and separated with a gradient of 70-98% mobile phase B over 4.6 minutes, followed by a 2 minute column wash at 98% mobile phase B. The column was then re-equilibrated to initial conditions. The analytical column temperature was maintained at 60 ˚C.
Multiple Reaction Monitoring (MRM) analyses were performed using a Xevo TQ-S micro Mass Spectrometer. All experiments were performed in positive electrospray ionization (ESI+) mode. The ion source temperature and capillary voltage were kept constant at 150 ˚C and 2.0 kV, respectively. The cone gas flow rate was 50 L/hr and desolvation temperature was 650 ˚C. All MRM transitions were monitored at a cone voltage of 40V and a collision energy of 25eV.
Method information was imported onto the LC-MS system using the Quanpedia functionality within MassLynx. This extendable and searchable database contains LC and MS methods as well as processing methods for use in TargetLynx for compound quantification.
|
LC system: |
ACQUITY UPLC I-Class FL |
|
Detection: |
Xevo TQ-S micro |
|
Column(s): |
CORTECS T3 2.7 μm (2.1 x 30 mm) |
|
Column temp.: |
60 ˚C |
|
Sample temp.: |
60 ˚C |
|
Injection volume: |
5 uL |
|
Flow rate: |
0.4 mL/min |
|
Mobile phase A: |
0.01% formic acid (aq) containing 0.2 mM Ammonium formate |
|
Mobile phase B: |
50% isopropanol in acetonitrile containing 0.01% formic acid and 0.2 mM Ammonium formate |
|
Gradient: |
After an initial 1.0 minute hold at 70% mobile phase B a linear gradient of 70-98% mobile phase B was applied over 4.6 minutes, followed by a 2 minute column wash at 98% mobile phase B and re-equilibration back to initial conditions |
|
MS system: |
Xevo TQ-S micro |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
2.0 kV |
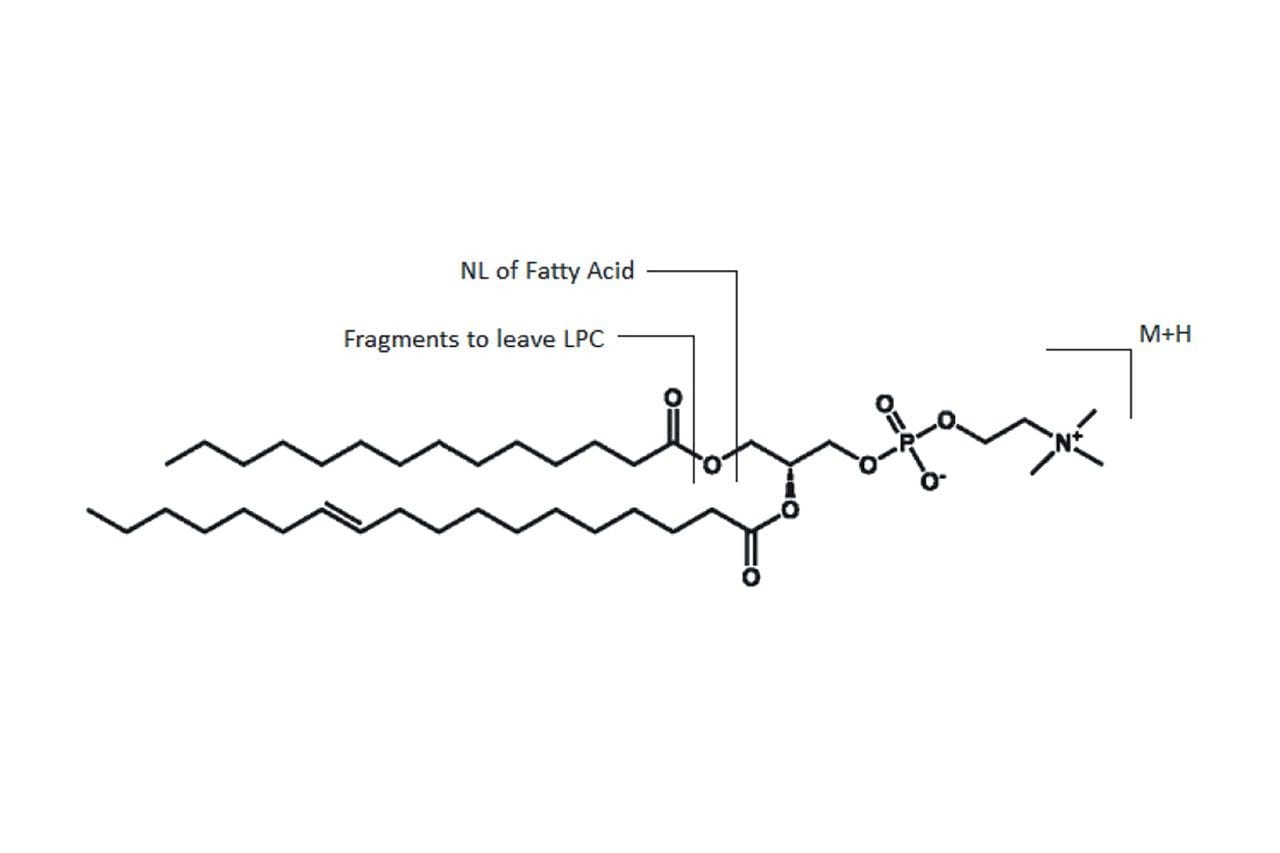
Diacyl-phosphatidylcholines (diacyl-PCs) were detected using a series of MRM transitions, where their precursor mass was the protonated ion [M+H]+ and the product ions were the neutral losses of either of the two fatty acid residues or fragmentation leaving one of the two corresponding Lyso-PCs due to the neutral loss of the fatty acids as alkyl ketenes (an example is given in Figure 2). Table 1 shows a list of the 57 individual diacyl-PC species that this method is capable of measuring. This quartet MRM approach allowed for the elucidation of the structural information for each PC’s lipid species. Figure 3 shows chromatograms acquired for 2 diacyl PCs containing 36 carbons and 4 double bonds within their 2 fatty acids. Figure 3 shows that as well as giving different specific MRM transitions, these 2 most common structurally isomeric species, PC(18:2_18:2) andPC(16:0_20:4), are also separated chromatographically.

A rapid UPLC-MS/MS methodology has been developed for the specific and semi-quantitative analysis of diacyl-phosphatidylcholine species in biomedical research lipidomics studies. This method has demonstrated to be suitable for the characterization of physiologically relevant levels of this important class of lipids in human serum. The method utilizes a single generic LC-MS platform that can be used for a variety of compound classes (including metabolomics, lipidomics, and proteomics). Deployment of this method in conjunction with other complementary methods available on the Waters Targeted Omics Method Library website (www.waters.com/targetedomics) can form the basis of a comprehensive suite of targeted multi-omic workflows.
720006935, June 2020