For research use only. Not for use in diagnostic procedures.
A rapid, targeted UPLC-MS/MS methodology has been developed for the semi-quantitative analysis of 14 endogenous steroids in human plasma/serum. This research method has demonstrated to be suitable for the analysis of these analytes at physiologically relevant levels in human plasma/serum. This MetaboQuan-R method is optimized on the same versatile LC-MS platform that has previously been utilized for the analysis of various classes of compounds including bile acids, amino acids, free fatty acids, tryptic peptides, acylcarnitines, tryptophan metabolites, and various lipids. This new steroids assay extends the MetaboQuan-R suite of methodologies, and allows the analysis of an expanding set of metabolites and lipids, when operated sequentially in a targeted multi-omics workflow.
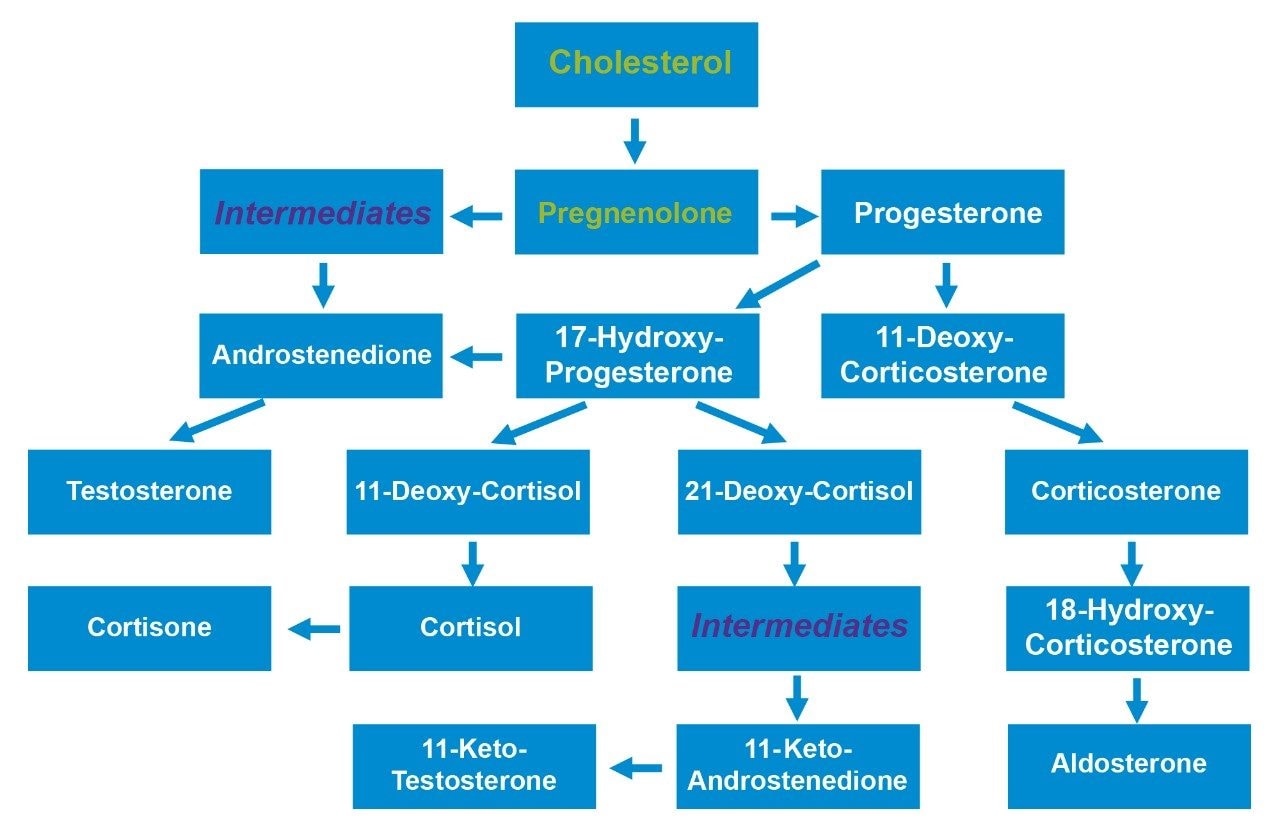
Steroid hormones and related compounds are extremely important, biologically active, molecules that are involved in countless essential biological functions (Figure 1). The analysis of these analytes is challenging since so many of them have similar chemical structures giving rise to multiple isobaric interferences. This chemical similarity means that chromatographic separation of these interfering (isobaric) species is essential for selective analysis. This is usually achieved with long chromatographic run times, derivatization or the use of multiple targeted assays.
Here we demonstrate a high-throughput UPLC-MS/MS research method for the semi-quantitative analysis of 14 steroids in human plasma/serum samples, without the need for derivatization, and using a generic, flexible LC-MS platform that has previously been used for multiple other endogenous compound classes (MetaboQuan-R). Thus enabling the high-throughput quantitative analysis of multiple metabolic classes in a single analytical run.
Human plasma/serum samples were prepared as described previously (Figure 1). Briefly, samples were prepared by liquid/liquid extraction using an ethyl acetate/hexane mixture. After evaporation, the resulting residue was solubilised in a methanol/water mixture prior to analysis by UPLC-MS/MS.
UPLC separation was performed with an ACQUITY UPLC I-Class System (Fixed Loop), equipped with a CORTECS T3 2.7 μm (2.1 x 30 mm) Column maintained at 60 ˚C. A 5 μL aliquot of the sample was injected onto the column and eluted under reversed-phase gradient conditions, where mobile phase A comprised 0.01% formic acid (aq) containing 0.2 mM Ammonium formate and mobile phase B comprised 50% isopropanol in acetonitrile containing 0.01% formic acid and 0.2 mM Ammonium formate. After an initial 2.7 minute hold at 15% mobile phase B, the steroids were eluted from the column and separated with a linear gradient of 15-40% mobile phase B over 4.3 minutes, followed by a 1 minute column wash at 98% mobile phase B at a flow rate of 0.45 mL/min. The column was then re-equilibrated to initial conditions.
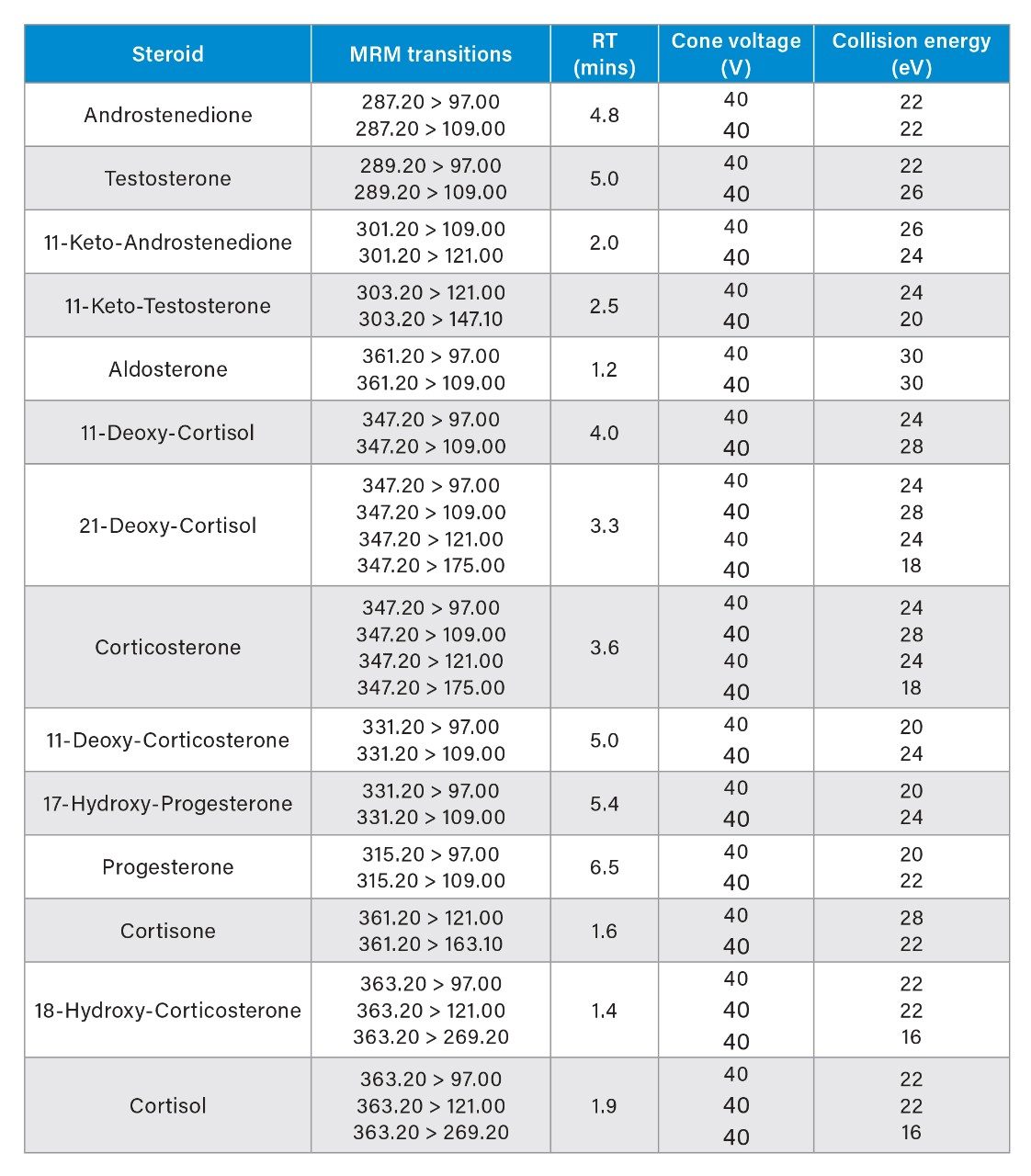
Steroids were detected using Multiple Reaction Monitoring (MRM) analyses on a Xevo TQ-S micro Mass Spectrometer. All experiments were performed in positive electrospray ionization (ESI+) mode. The ion source temperature and capillary voltage were kept constant and set to 150 ˚C and 2.0 kV respectively. The cone gas flow rate was 50 L/hr and desolvation temperature was 650 ˚C. The MRM transitions employed are detailed in Table 1.
Method information was imported into the LC-MS system using the Quanpedia functionality within MassLynx. This extendable and searchable database produces LC and MS methods as well as processing methods for use in TargetLynx for compound quantification.
|
LC system: |
ACQUITY UPLC I-Class FL |
|
Detection: |
Xevo TQ-S micro |
|
Column(s): |
CORTECS T3 2.7 μm (2.1 x 30 mm) |
|
Column temp.: |
60 ˚C |
|
Sample temp.: |
6 ˚C |
|
Injection volume: |
5 uL |
|
Flow rate: |
0.45 mL/min |
|
Mobile phase A: |
0.01% formic acid (aq) containing 0.2 mM Ammonium formate |
|
Mobile phase B: |
50% isopropanol in acetonitrile containing 0.01% formic acid and 0.2 mM Ammonium formate |
|
Gradient: |
After an initial 2.7 minute hold at 15% mobile phase B a linear gradient of 15-40% mobile phase B was applied over 4.3 minutes, followed by a 1 minute column wash at 98% mobile phase B and re-equilibration back to initial conditions |
|
MS system: |
Xevo TQ-S micro |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
2.0 kV |
The 14 steroids detailed in Table 1 were extracted, separated, and detected using the UPLC-MS/MS platform and extraction protocol described. Within this list of 14, there are 5 sets of compounds (see list below) that, due to structural similarity are isobaric, and as a result interfere with each other in the mass spectrometer.
Set A – Testosterone and Androstenedione
Set B – 11-Keto-Testosterone and 11-Keto-Androstenedione
Set C – Aldosterone, Cortisone, 18-Hydroxy-Corticosterone, and Cortisol
Set D – Corticosterone, 11-Deoxy-Cortisol, and 21-Deoxy-Cortisol
Set E – 11-Deoxy-Corticosterone and 17-Hydroxy-Progesterone
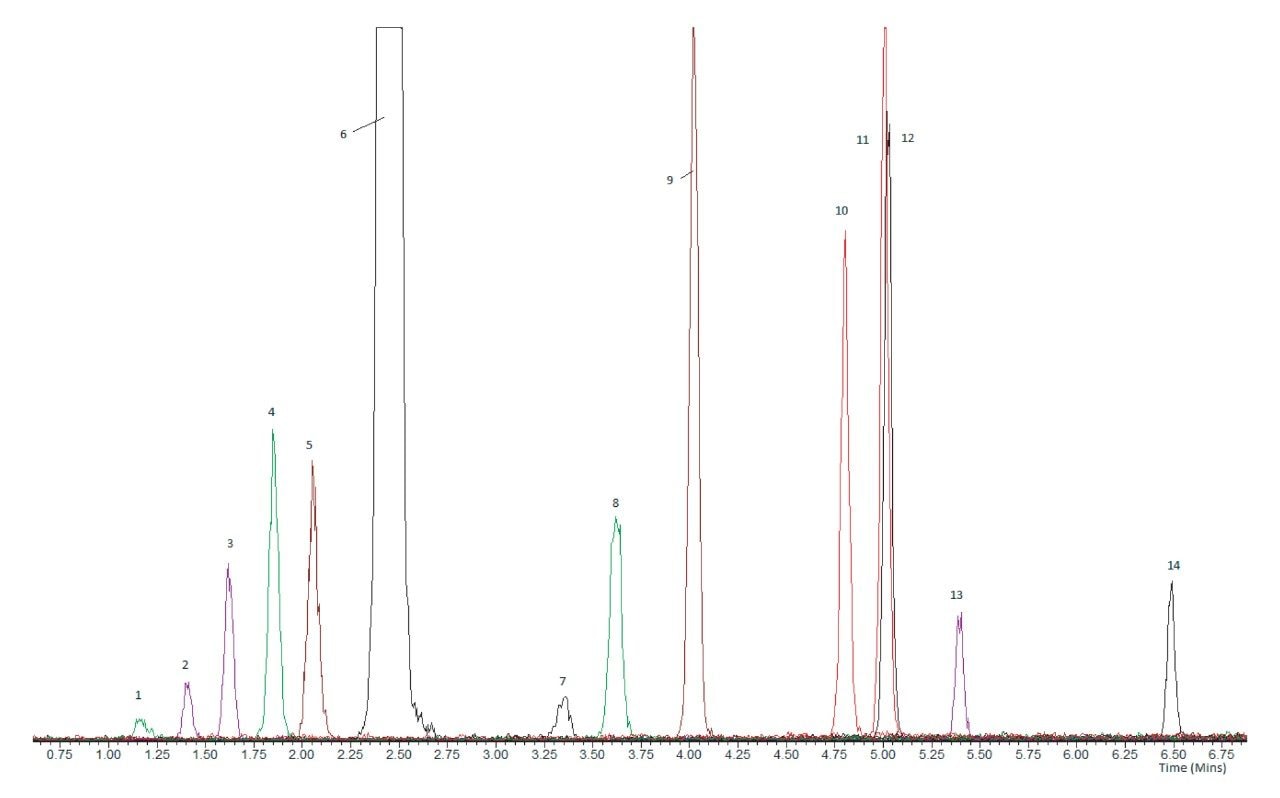
In order to selectively analyze all 14 steroids, it is necessary to chromatographically resolve these sets of isobaric steroids. The UPLC separation developed using the MetaboQuan-R platform successfully separated these 5 sets of steroids from each other, such that specific analysis can be achieved for all analytes. Figure 2 shows the UPLC separation for the 14 steroids, including these 5 key isobaric steroid sets.
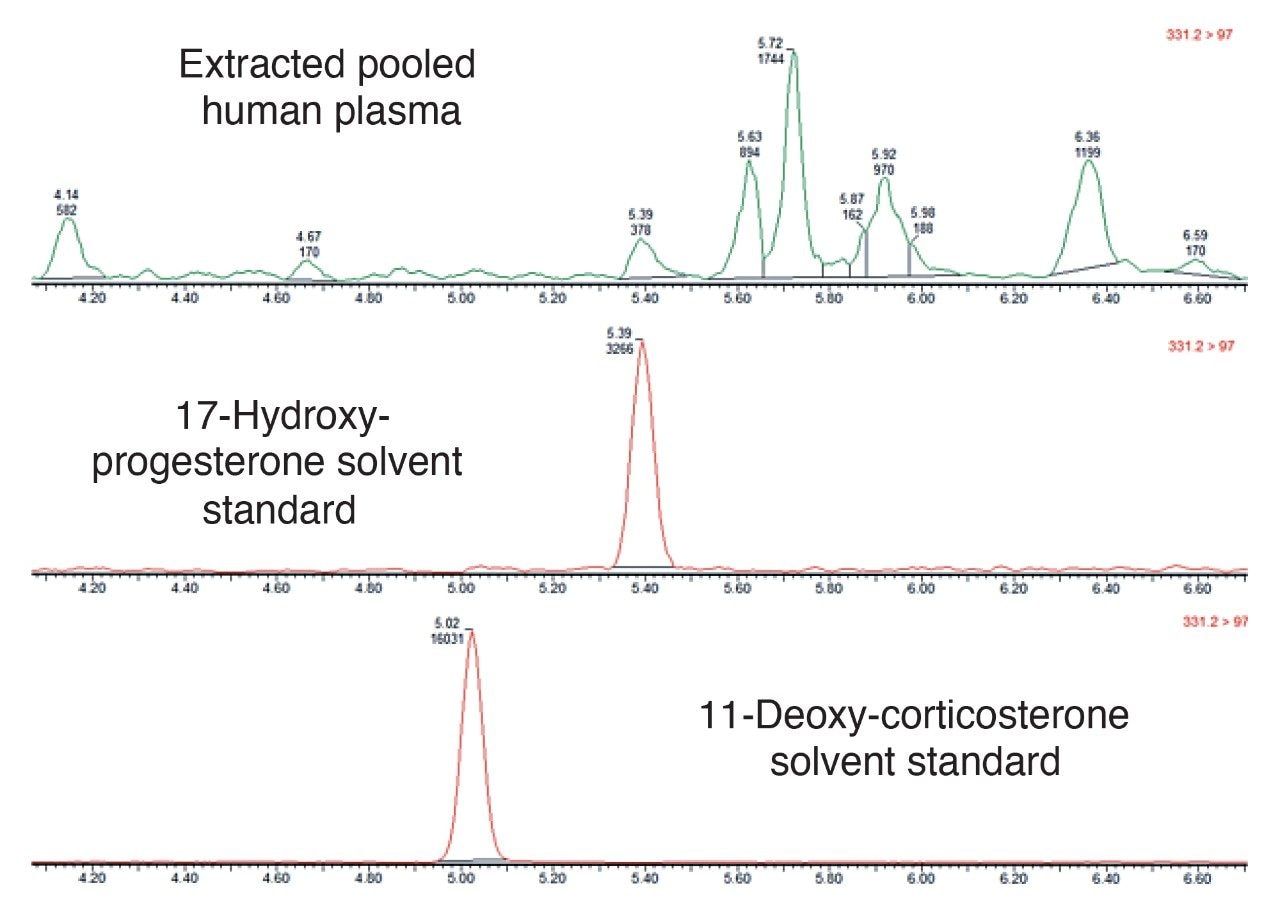
The chromatograms shown in Figure 3 demonstrate the isobaric interference between the two steroids 17-Hydroxy-Progesterone and 11-Deoxy-Corticosterone. Here we can clearly see that both steroids have the same precursor and product ions (MRM transition 331.2 > 97.0). The uppermost chromatogram in Figure 3 is from an extracted pooled human plasma sample, which shows the peak for 17-Hydroxy-Progesterone along with other background peaks. This illustrates the need for a good chromatographic resolution for steroid analysis. This MetaboQuan-R chromatographic method not only successfully separates 17-Hydroxy-Progesterone peak from another interfering steroid of interest, 11-Deoxy-Corticosterone, but also separates it from at least 6 other unknown endogenous isobaric interferences.
A rapid UPLC-MS/MS methodology has been developed for the research analysis of 14 endogenous steroids in human plasma and serum. This method demonstrated to be suitable for the analysis of these steroids at physiologically relevant levels in human plasma and serum. This method utilizes a flexible reversed-phase UPLC-MS/MS platform that can be used for various compound classes (including bile acids, free fatty acids, amino acids, tryptic peptides, acyl-carnitines, and lipids); thus allowing the assay to be employed as part of a suite of methodologies that can be operated sequentially as part of a targeted multi-omics workflow with broad compound coverage.
720006936, June 2020