Analysis of Sugar Alcohols and Allulose Using an Arc HPLC System with Refractive Index Detection
Abstract
Overconsumption of sugar (sucrose) can lead to many health issues such as obesity, diabetes, dental diseases, and ADHD (attention-deficit/hyperactivity disorder). To support the reduction of these problems, many food and beverage manufacturers have reformulated or developed new products with sugar substitutes such as sugar alcohols (polyols) or allulose, an epimer of the monosaccharide sugar fructose, which has a lower glycemic index and calories than sugar when digested. There is no fixed daily intake, but it is important to know the value of sugar alcohols and allulose present in food and pharmaceutical products to prevent overconsumption, due to possible laxative effects from some of the sugar alcohols. An analytical method has been developed to detect and quantify sugar alcohols and allulose in beverages and other low-calorie products. Arc HPLC-RI was combined with an Atlantis Premier BEH Z-HILIC analytical column, which provides an increase in polar analyte retention and offers a different selectivity when compared to other hydrophilic interaction liquid chromatography (HILIC) chemistries. The proposed analytical workflow may be suitable for supporting manufacturers and contract testing laboratories in standardizing analyses for sugar alcohols and allulose in sugar-free or reduced-sugar products.
Benefits
- Retention and separation of 7 sugar alcohols and allulose using the Atlantis Premier BEH Z-HILIC Column, which is efficient for products using blends of sweeteners
- Simple isocratic liquid chromatography (LC) method, ease of method setup for routine product quality control
- Arc HPLC cycle injector valve reduces downtime and method risks due to possible precipitation of samples
- RI Detection of components without UV chromophores
- Excellent reproducibility, accuracy, and precision of Arc HPLC-RI Detector with the Atlantis Premier BEH Z-HILIC Column
Introduction
Sugar alcohols, or polyols, are derived from carbohydrates in which the aldehyde or ketone group is reduced and converted into alcohol.1 Sugar alcohols are sweeteners and have fewer calories than regular sugars. Sugar alcohols can be found naturally in fruits like apples, bananas, peaches, pears, apricots, and oranges, as well as being added to baked goods and beverages. Sugar alcohols do not have any fixed daily intake. However, overconsumption of sugar alcohols can result in diarrhoea and disturb the blood sugar level. Therefore, consumers should receive accurate information about the number of sugar alcohols present in various food items.2,3 Food manufacturers have introduced sugar substitutes, such as sugar alcohols and allulose to some low-calorie products to help reduce the consumers intake of sugar, to support the reduction of obesity levels.
Allulose is an epimer of the monosaccharide fructose and is used by food and beverage manufacturers as a low-calorie sweetener.4 Allulose is found in small quantities in wheat, raisins, figs, and molasses. The United States Food and Drug Administration (FDA) replied with no objections to three Generally Recognized as Safe (GRAS) notifications on the use of allulose as a sugar substitute. In 2020, the FDA provided guidance on the declaration of allulose and calorie information on nutritional and supplement facts labels.5 Allulose is not approved for use as a sugar substitute in the European Union (EU) or the United Kingdom (UK). Allulose is classified as a novel food and requires market authorization and a safety review by the European Food Safety Authority (EFSA) and the UK Food Safety Authority (UKFSA).6
Sugar alcohols and allulose have no chromophore and therefore can be measured using detectors such as refractive index (RI), evaporative light scattering (ELS), or mass spectrometry (MS). This application note will outline an example analytical method to quantify sugar alcohols and allulose in various low-calorie products. The separation was performed on an Arc HPLC-RI combined with an Atlantis Premier BEH Z-HILIC analytical column.
Experimental
Materials and Reagents
Standards and buffer salts.
Sugar alcohols (erythritol, glycerol, maltitol, mannitol, xylitol, sorbitol) were obtained from Sigma-Aldrich; allulose (Psicose) standards were obtained from Fisher Scientific.
Reagents
The acetonitrile was obtained from Honeywell Research Chemicals.
Products containing the target sweeteners were purchased from local and on-line retailers.
Sample Preparation
Standards preparation:
Sugar alcohols and allulose standards were prepared at a concentration of 100 mg/mL in water and stored at 4 °C. A mixed standard of sugar alcohols and allulose was prepared at 5 mg/mL via serial dilution in 50:50 acetonitrile:water. The calibration curves ranged from 0.16 to 5 mg/mL.
Sample products:
Three replicates of gum, children’s cold medicine, and an allulose powdered drink mix were weighed out (1 g) and dissolved in 20 mL water. 100 µL solution was diluted with 400 µL of high performance liquid chromatography (HPLC) water, vortexed and 500 µL acetonitrile was added.
Beverages were filtered with a 0.2 μm PVDF syringe filter. 100 µL samples were diluted with 400 µL water, vortexed and 500 µL acetonitrile was added into the samples. 5 µL were injected.
LC Conditions
|
LC system: |
Arc HPLC System |
|
Detection: |
RI 2414 (Sampling rate 10 points/sec) |
|
Vials: |
LCGC Certified Clear Glass, Max Recovery, with Cap and Preslit PTFE/Silicone Septum, 1.5 mL (p/n: 186000327C) |
|
Filter: |
Syringe Filter 0.2 µm PVDF (p/n: WAT200806) |
|
Column(s): |
Atlantis Premier BEH Z-HILIC, 4.6 x 150 mm, 2.5 µm (p/n: 186009994) |
|
Column temp.: |
55 °C |
|
Sample temp.: |
25 °C |
|
Detector temp.: |
55 °C |
|
Injection volume: |
5 µL |
|
Flow rate: |
0.3 mL/min |
|
Mobile phase: |
75:25 Acetonitrile:water |
|
Sample diluent: |
50:50 Acetonitrile:water |
|
Seal wash: |
5:95 Acetonitrile:water |
|
Needle wash: |
5:95 Acetonitrile:water |
|
Purge solvent: |
50:50 Acetonitrile:water |
Data Management
|
Chromatography software: |
Empower 3 Chromatography Data Software (CDS) |
Results and Discussion
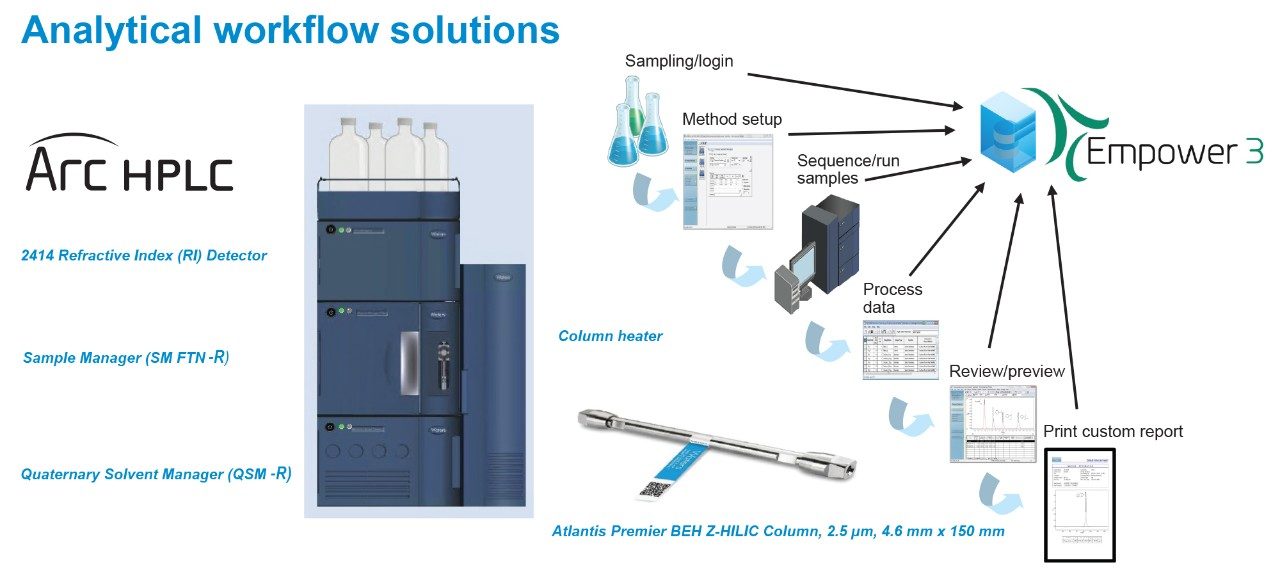
The analysis of sugar alcohols and allulose was performed using an Arc HPLC with a 2414 Refractive Index (RI) Detector. RI detection requires the use of an isocratic solvent mobile phase, which can result in challenges to separate the sugar alcohols and allulose in a single method. The Atlantis Premier BEH Z-HILIC Column that was utilized offers an increase in analyte retention of highly polar compounds; it provided a different selectivity compared to other HILIC column stationary phases, allowing the separation of erythritol, allulose, sorbitol, and mannitol. The column has shown good peak shape and resolution of sugar alcohols and allulose (Figure 1). The Empower 3 Chromatography Data Software has been used for instrument control, acquisition, and processing data. Figure 2 shows the analytical workflow to analyze sugar alcohols and allulose.
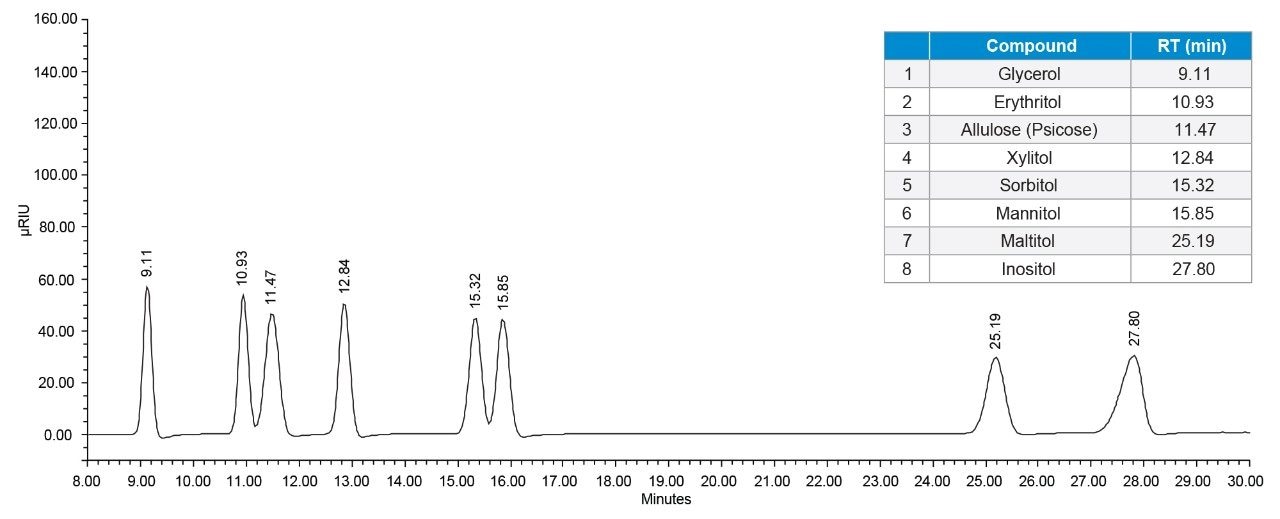
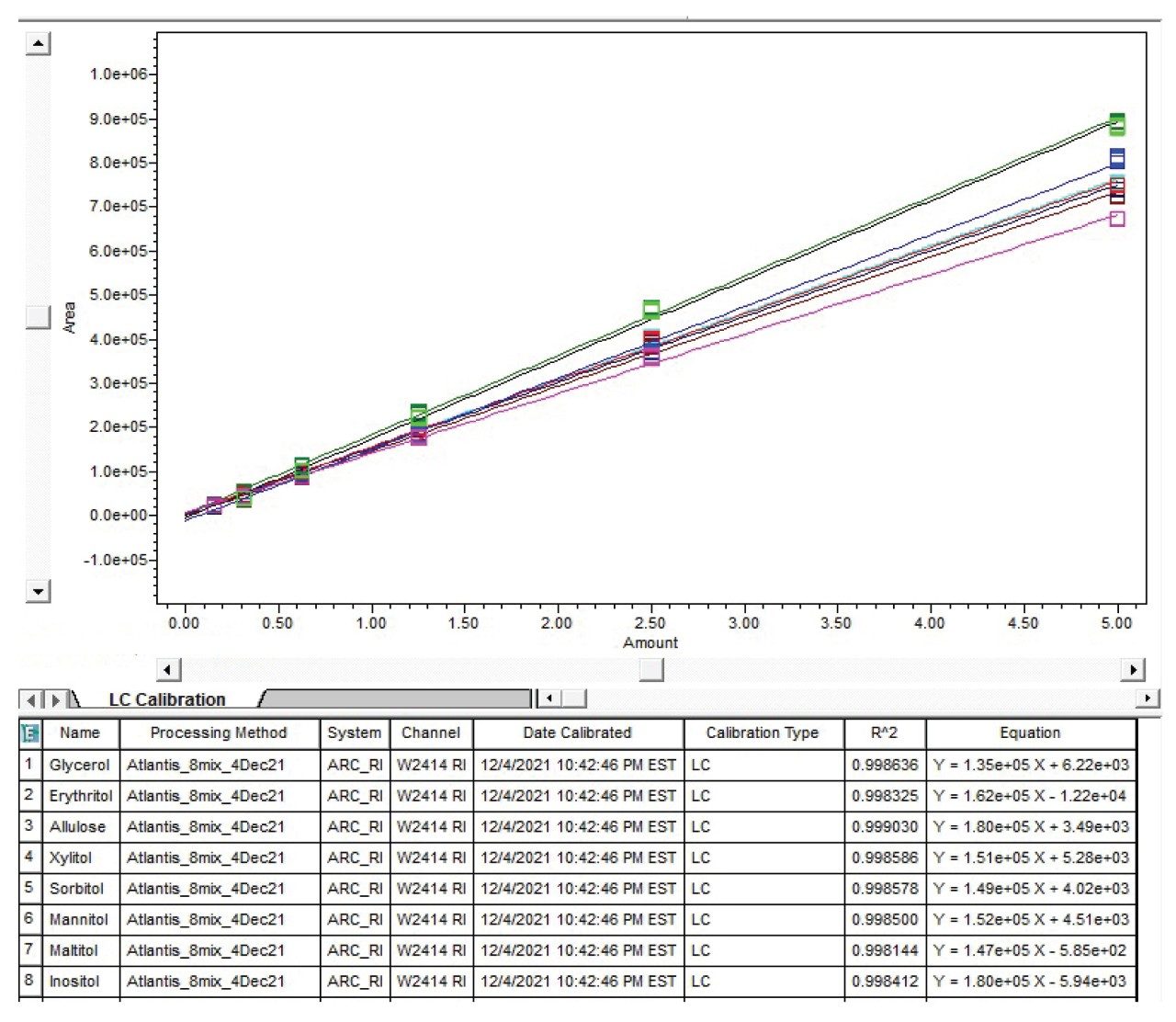
Calibration curve: Linearity, precision of area, and retention time
Multi-point calibration curves for sugar alcohols and allulose were prepared via serial dilution in 50:50 acetonitrile and water. Calibration curves were generated from 0.16 to 5 mg/mL and showed good linearity (R2 >0.998). Calibration curves of sugar alcohols and allulose are shown in Figure 3.
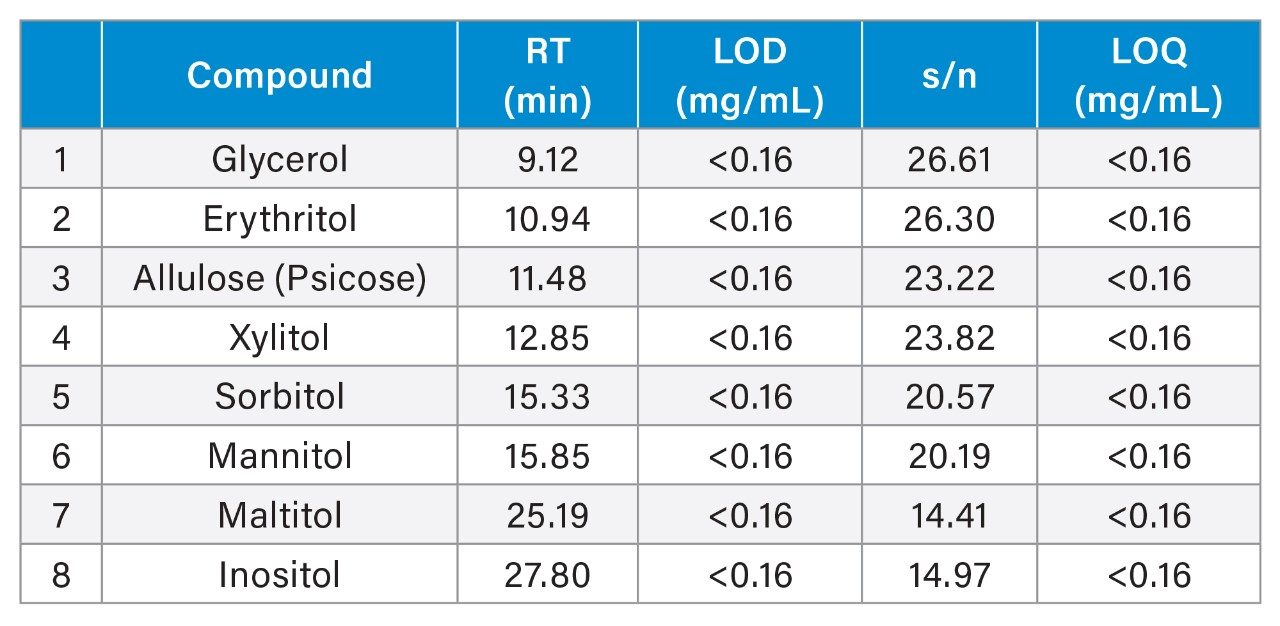
The signal-to-noise (s/n) based on peak-to-peak measurement of the sugar alcohols and allulose was used to calculate the limit of detection (LOD) (3:1) and limit of quantitation (LOQ) (10:1) in the Empower Software. Table 1 provides a summary of the LOD and LOQ of sugar alcohols and allulose.
Method robustness
Column performance and pressure trace
Samples were run using a high percentage of acetonitrile in the mobile phase, which can cause some solubility issues and sample precipitation in the injector valve, potentially increasing the overall system pressure. The Arc HPLC has a cycle injector valve in the Sample Manager (SM FTN-R) that can be included in the method to help prevent blockages and carry-over issues with complex matrix samples. The pressure trace of the column was monitored for 150 injections. There were no significant changes across the pressure trace (less than 10 psi), as shown in Figures 4A and 4B.
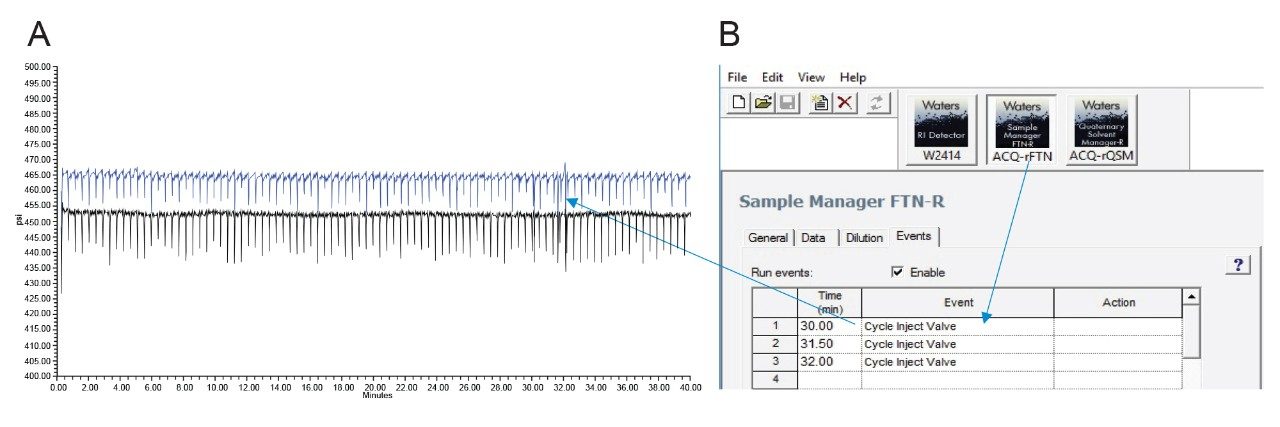
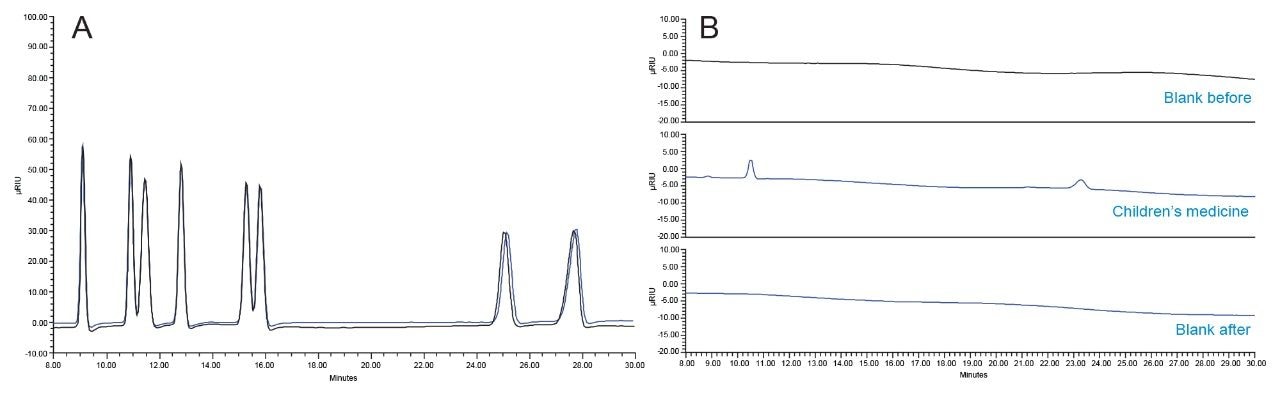
The reproducibility of the retention time from the column was monitored for 150 injections from the first injection of 5 mg/mL standards overlaid with the last injection of a 5 mg/mL standard, interspersed with matrix samples (Figure 5A). Figure 5B shows the chromatograms of blank injections that were injected before and after three injections of the children's medicine sample. There was no carryover observed from these samples.
Analysis of sugar alcohols and allulose containing products:
The quantitative results generated from the analysis of sugar alcohol and allulose containing products are shown in Table 2.
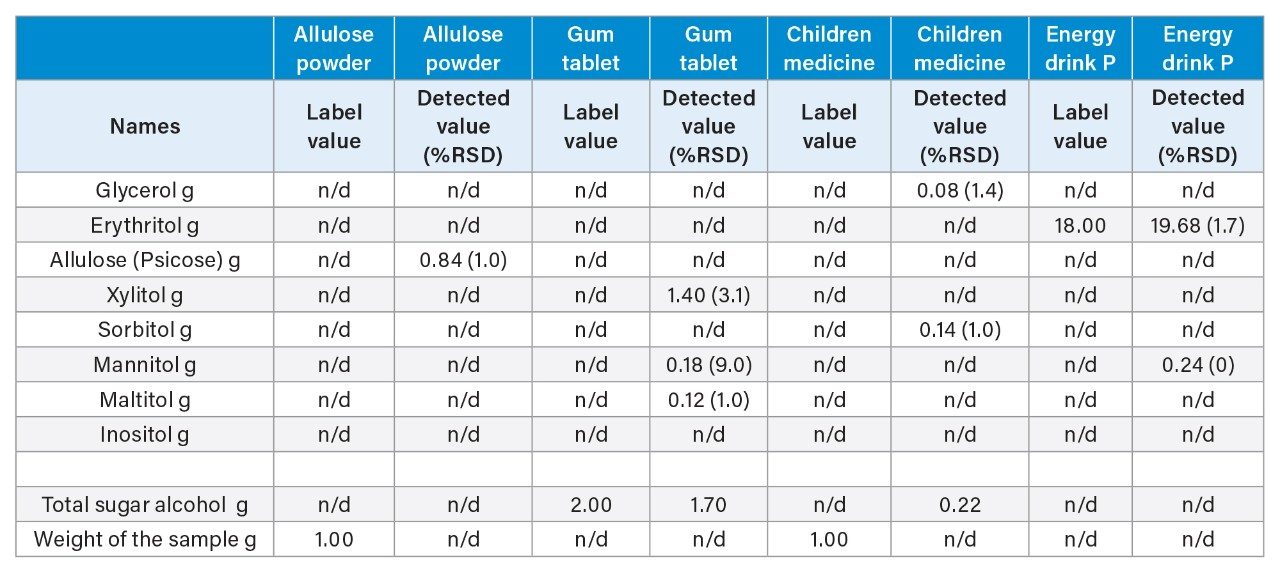
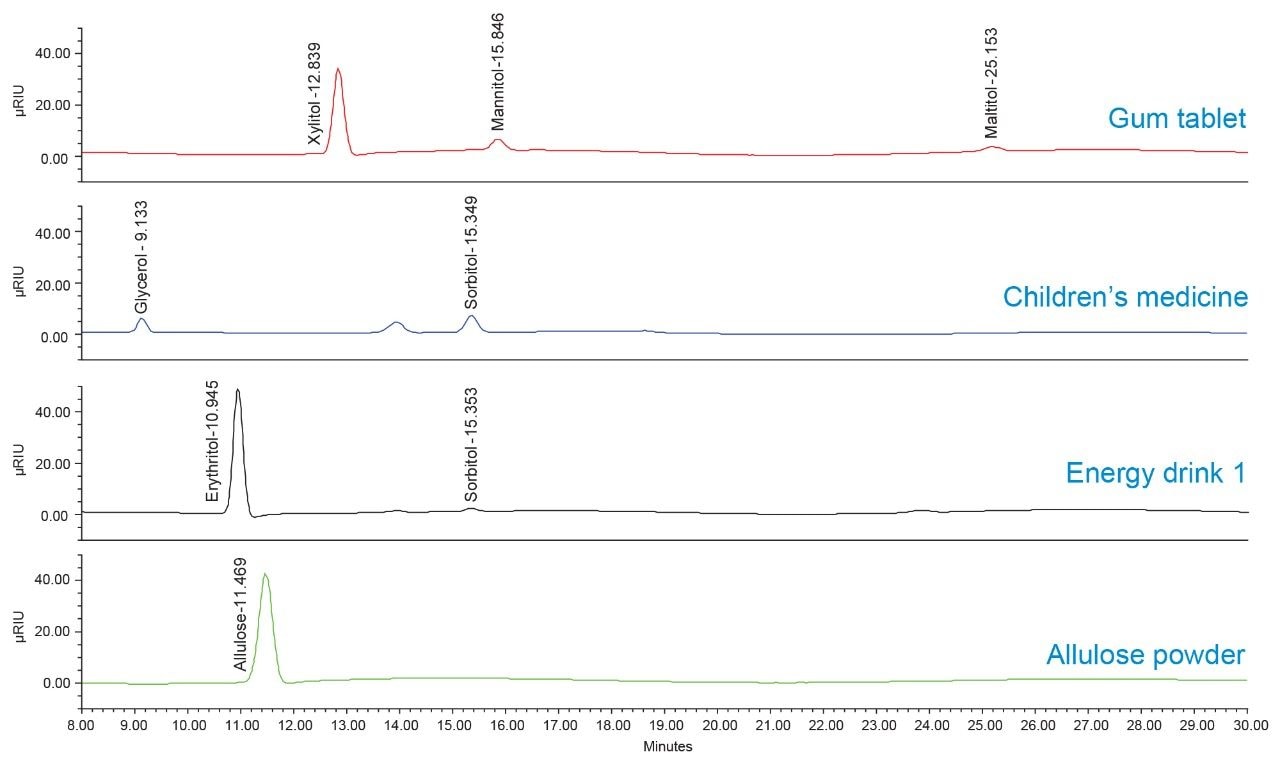
In fruit energy drink P sample, 19.7 g of erythritol was detected. The label claimed 18 g of total carbohydrate and 2 g of sugar without the value of erythritol.
In the gum tablets, 1.7 g of the detected sugar alcohols was the sum of xylitol, mannitol, and maltitol in the tablet, the label claim was 2 g of total sugar alcohol for each tablet.
In the children’s medicine, 0.22 g of glycerol and sorbitol were detected per 1 g of the sample.
In the allulose powdered mix drink, 0.84 g of allulose was detected per 1 g of the sample.
A representative chromatogram of the analysis of sugar alcohol and allulose products is shown in Figure 6.
Conclusion
- The Waters Arc HPLC-RI System combined with the Atlantis Premier BEH Z-HILIC Column enabled the separation of sugar alcohols and allulose.
- Sugar alcohols and allulose are separated using a simple isocratic method for detection by RI.
- Atlantis Premier BEH Z-HILIC Columns provide an increase in polar analyte retention and offer a different selectivity when compared to other HILIC chemistries.
- This analytical workflow is suitable for supporting manufacturers in standardizing analyses for sugar alcohols and allulose in food and beverages.
References
- Wolever Th, A. Piekarz, M. Hollands, K. Younker, Rapaille. A, Goosens. J, etc. 2002. Sugar Alcohols and Diabetes: A Review. Canadian Journal of Diabetes.26(4): 356–362.
- Rapaille. A, Goosens. J, & Heume. M. (2016) Sugar alcohols. Encyclopedia of Food and Health, 211–216.
- Ruskone-Fourmestraux. A, Attar. A, Chassard. D, Coffin. B, Bornet. F, Bouhnik. Y. (2003) A Digestive Tolerance Study of Maltitol after Occasional and Regular Consumption in Healthy Humans. European Journal of Clinical Nutrition, 57, 26–30.
- Akram Hossain; Fuminori Yamaguchi; Tatsuhiro Matsuo; Ikuko Tsukamoto; Yukiyasu Toyoda; Masahiro Ogawa; Yasuo Nagata; Masaaki Tokuda. (November 2015) "Rare Sugar d-allulose: Potential Role and Therapeutic Monitoring in Maintaining Obesity and Type 2 Diabetes Mellitus". Pharmacology & Therapeutics. 155: 49–59.
- U.S. FDA (2020) Guidance for Industry: The Declaration of Allulose and Calories from Allulose on Nutrition and Supplement Facts Labels. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-declaration-allulose-and-calories-allulose-nutrition-and-supplement-facts-labels.
- Southey, F. (2021) Allulose approval in Europe to be sought by New Ingredients Consortium. Food Navigator. https://www.foodnavigator.com/Article/2021/12/07/Allulose-approval-in-Europe-to-be-sought-by-new-ingredients-consortium.
720007499, January 2022