Determination of Pesticide Residues in Rice-Based Baby Food Using GC-MS/MS with APGC™ After Extraction and Clean Up Using QuEChERS
This is an Application Brief and does not contain a detailed Experimental section.
Abstract
Reliable analytical methods are needed for detection, quantification, and identification of hundreds of pesticide residues in many different commodities. This application brief describes the development and validation of a comprehensive method based on gas chromatography with tandem mass spectrometry (GC-MS/MS) for the determination of 166 pesticides in rice-based baby food. Extracts were prepared using a published version of QuEChERS for cereals followed by determination with GC-MS/MS. The use of GC-MS/MS utilizing atmospheric pressure ionization (APGC) has been shown to offer significant improvements in performance over electron ionization (EI) for pesticide residue analysis, in terms of selectivity, specificity, and speed of analysis. The extremely high sensitivity of the APGC Xevo™ TQ-XS System was demonstrated with reliable detection for almost all analytes at concentrations as low as 0.0003 mg/kg, even when injection volume was limited to 1 µL. The method was successfully validated in rice-based baby food using the SANTE guidelines document. The results from analysis of the spikes at both concentrations showed that 91% and 98% of the analytes were within the required tolerances for recovery and repeatability, respectively. The method is considered sensitive, specific, accurate, and suitable for the determination of residues of a wide range of GC-amenable pesticides for checking compliance with the specific maximum residue levels (MRLs) set for food intended for infants and young children and has the potential for determination at much lower concentrations.
Benefits
- The method can generate extremely high sensitivity to meet the regulatory limits required for the determination of pesticide residues in baby foods
- The method is also suitable for determination at lower concentrations as typically required by the food industry and for risk assessment purposes
- Sensitivity was achieved using conventional splitless injection of 1 µL of acetonitrile extract
Introduction
Pesticide residues resulting from the use of plant protection products on crops that are used for food production may pose a risk factor for public health. Infants and young children are considered as a vulnerable group when it comes to exposure to pesticide residues in foods as they have higher relative food intake to body weight than adults, diet is less varied and internal organs and central nervous system are still in development. In the US, tolerances for pesticides are set by the EPA for a range of in foodstuffs and are not a part of US infant formula legal requirements. In Europe, specific MRLs were set for food intended for infants and young children. Commission Directive 2006/125/EC specifically applies to processed cereal-based foods and baby foods for infants and young children and Commission Delegated Regulation (EU) 2021/1041 amending Delegated Regulation (EU) 2016/127 deals with the requirements for pesticides in infant formulae and follow-on formulae.1,2 Following the precautionary principle, the legal limits for these types of food products were set at very low levels. In general, the default MRL of 0.01 mg/kg is applicable but more severe limitations were set for pesticides or metabolites of pesticides with an ADI lower than 0.0005 mg/kg body weight per day. Certain pesticides have MRLs listed at lower concentration (0.004–0.008 mg/kg) and others should not be used at all in agricultural production intended for infant formula and baby food. These analytes need to be tested down to a reporting limit of at least 0.003 mg/kg.
Compliance with these MRLs is checked by the monitoring for residues in various types of food specifically dedicated to children using suitable validated analytical methods including the use of multi-residue approaches. These are often based on the combination of the QuEChERS sample preparation and liquid chromatography with tandem mass spectrometry (LC-MS/MS) and GC-MS/MS determinative steps. Both LC-MS/MS and GC-MS/MS techniques are required given the different physico-chemical properties of the analytes to be sought. Tandem mass spectrometric detection provides the sensitivity and selectivity needed to determine residues at the very low concentrations mandated and to ensure the baby food is compliant. Methods often need to be sensitive enough to determine residues accurately well below the specified legal limits to generate data for risk assessment purposes. Governments typically operate pesticide residue testing programs, and the food industry also carries out its own testing.
The use of GC-MS/MS utilizing atmospheric pressure ionization (APGC) has been shown to offer significant improvements in performance over EI for pesticide residue analysis, in terms of selectivity, specificity, and speed of analysis.3 We recently demonstrated the performance of a method for the determination of pesticide residues in cucumber using GC-MS/MS with APGC on Xevo TQ-XS after QuEChERS.4 The objective of this study was to demonstrate the performance of a method for the determination of residues of pesticides and their metabolites, at concentrations suitable for checking MRL compliance in baby foods and lower, using GC-MS/MS with APGC on Xevo TQ-XS. The validation batch was prepared by the European Union Reference Laboratory on Pesticide Residues in Cereals and Feeding Stuff (EURL CF) within the National Food Institute Technical University of Denmark. Samples were extracted using a modification of the CEN QuEChERS method designed for analysis of cereals.5
Results and Discussion
Validation was performed by replicate analysis of spiked test portions of a rice-based baby food. Extracts were analyzed by GC-MS/MS, with APGC on Xevo TQ-XS, using previously published conditions.4 The following factors were assessed: selectivity, sensitivity, calibration graph characteristics, recovery, and within-laboratory repeatability (RSDr). Recovery and repeatability were determined from the analysis of six replicates prepared at two concentrations: 0.0005 and 0.001 mg/kg.
The chromatograms for flufenoxuron and pymetrozine both exhibited significant peak tailing and those for carbaryl were impacted by significant isobaric interference on both MRM transitions. Carbosulfan exhibited good calibration but was not detected in the spiked samples. Carbosulfan tends to rapidly degrade in acidic extracts, typically to carbofuran. Dicofol and tolylfluanid were not detected in the matrix-matched standards or spikes, presumably due to stability issues. The degradants for each pesticide, 4,4’-dichlorobenzophenone (DBP) and dimethylsulfotoluidid (DMST), were monitored to represent these analytes instead. Results are given for all remaining 166 analytes.
The gas chromatographic method provided separation of the target analytes from isobaric interferences derived from the matrix for all but carbaryl. Chromatograms for the main MRM transitions for a selection of analytes are shown in Figure 1.
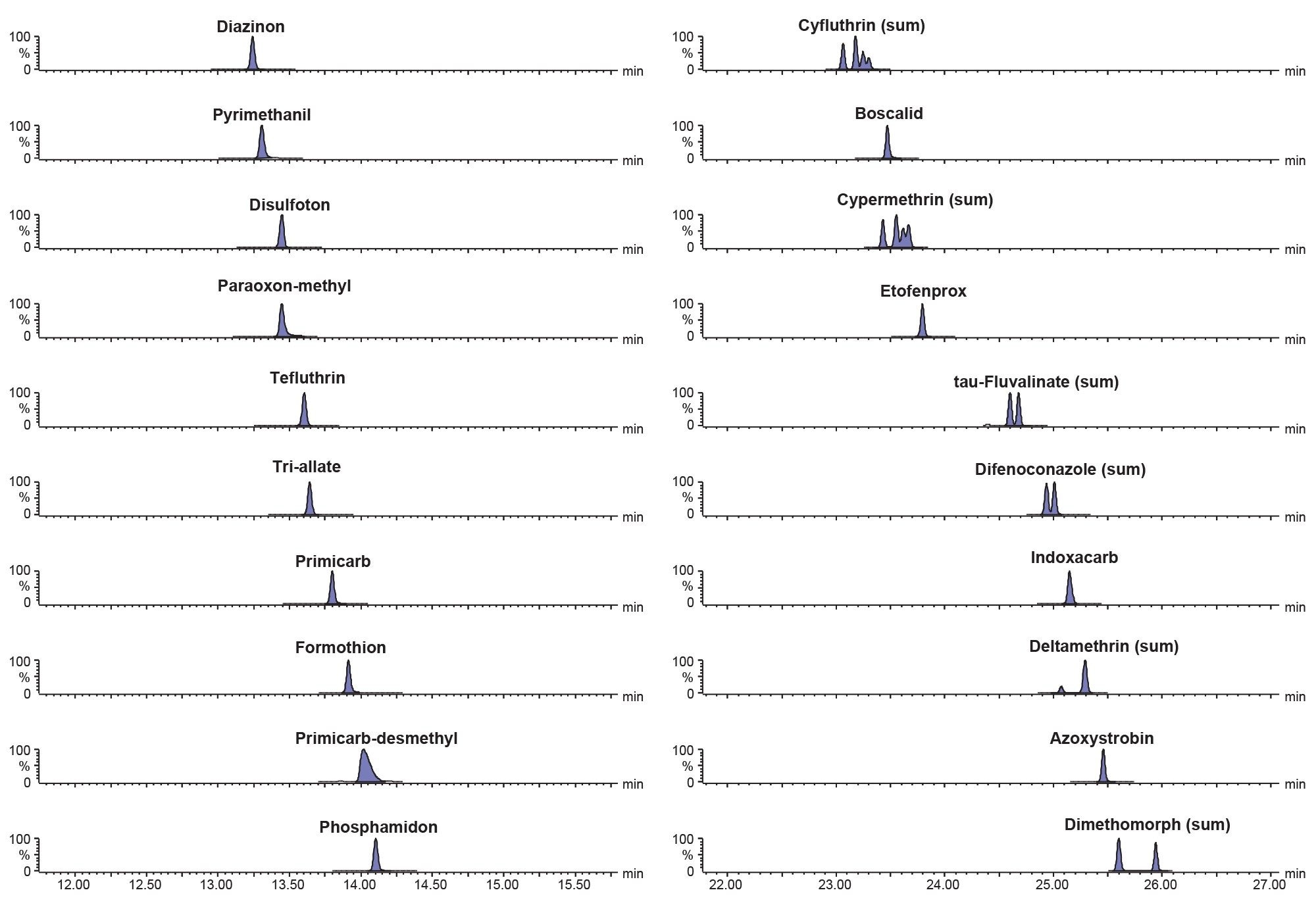
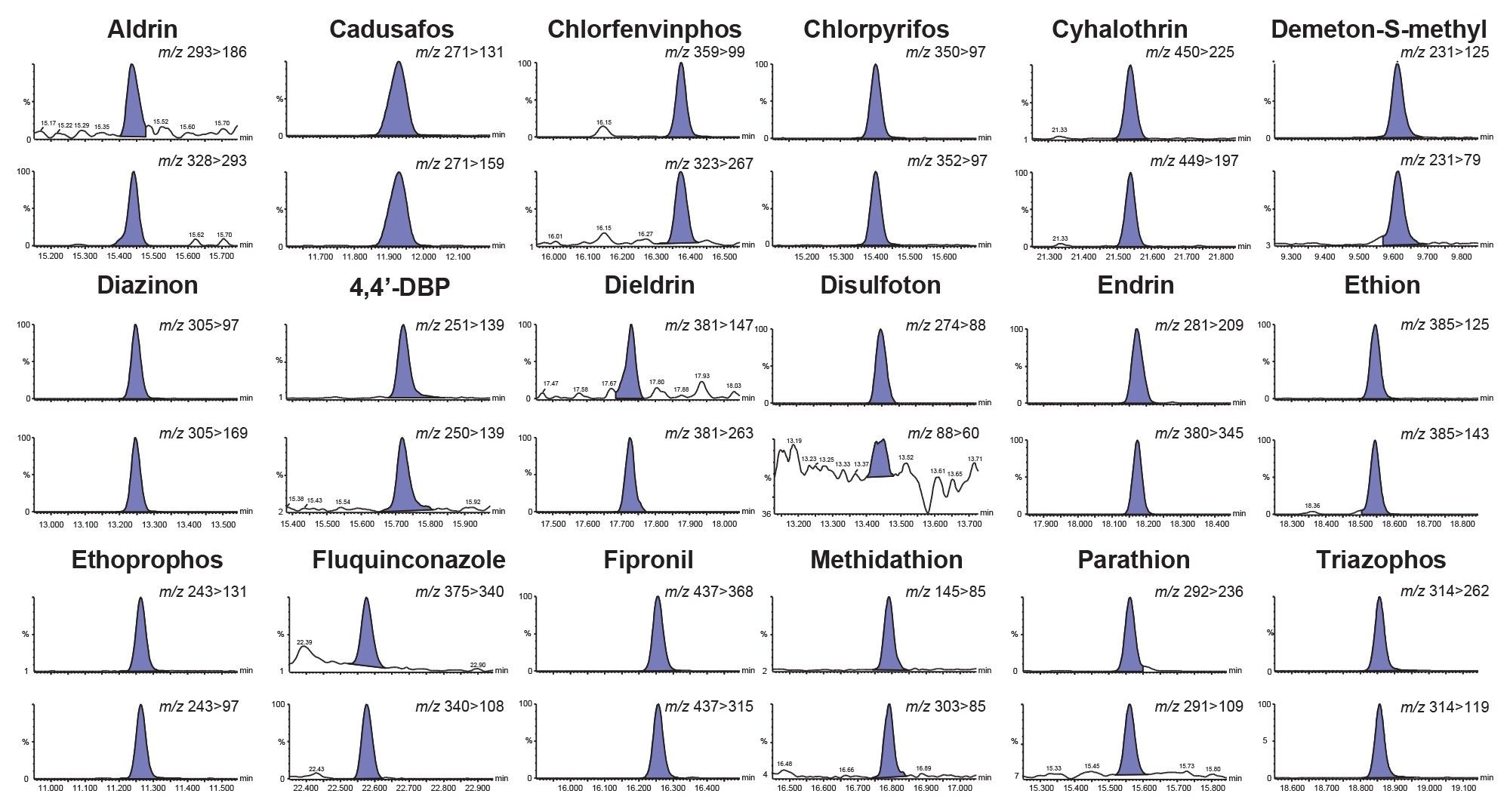
The sensitivity of the method was evaluated by assessment of the response of the matrix-matched standard at the lowest concentration prepared (0.0003 mg/kg) and consideration of the response from the blank. The blank baby food was found to contain a residue of 2-phenylphenol but not at a high enough concentration to comprise quantification. As blank values should not be higher than 30% of the residue level corresponding to the reporting limit (RL), the RL was raised to a value of 0.001 mg/kg and so results obtained from the analysis of the spikes can only be considered indicative. Of the 165 remaining analytes (see Annex for full list), all but one could be detected at 0.0003 mg/kg. The limit of detection (LOD) for thiometon was 0.0005 mg/kg. This demonstrates the extremely high sensitivity of the APGC approach with reliable detection for almost all the analytes at concentrations as low as 0.0003 mg/kg even when injection volume was limited to 1 µL. Figure 2 shows chromatograms from the analysis of a selection of priority pesticides in the baby food matrix-matched standard at 0.0005 mg/kg.
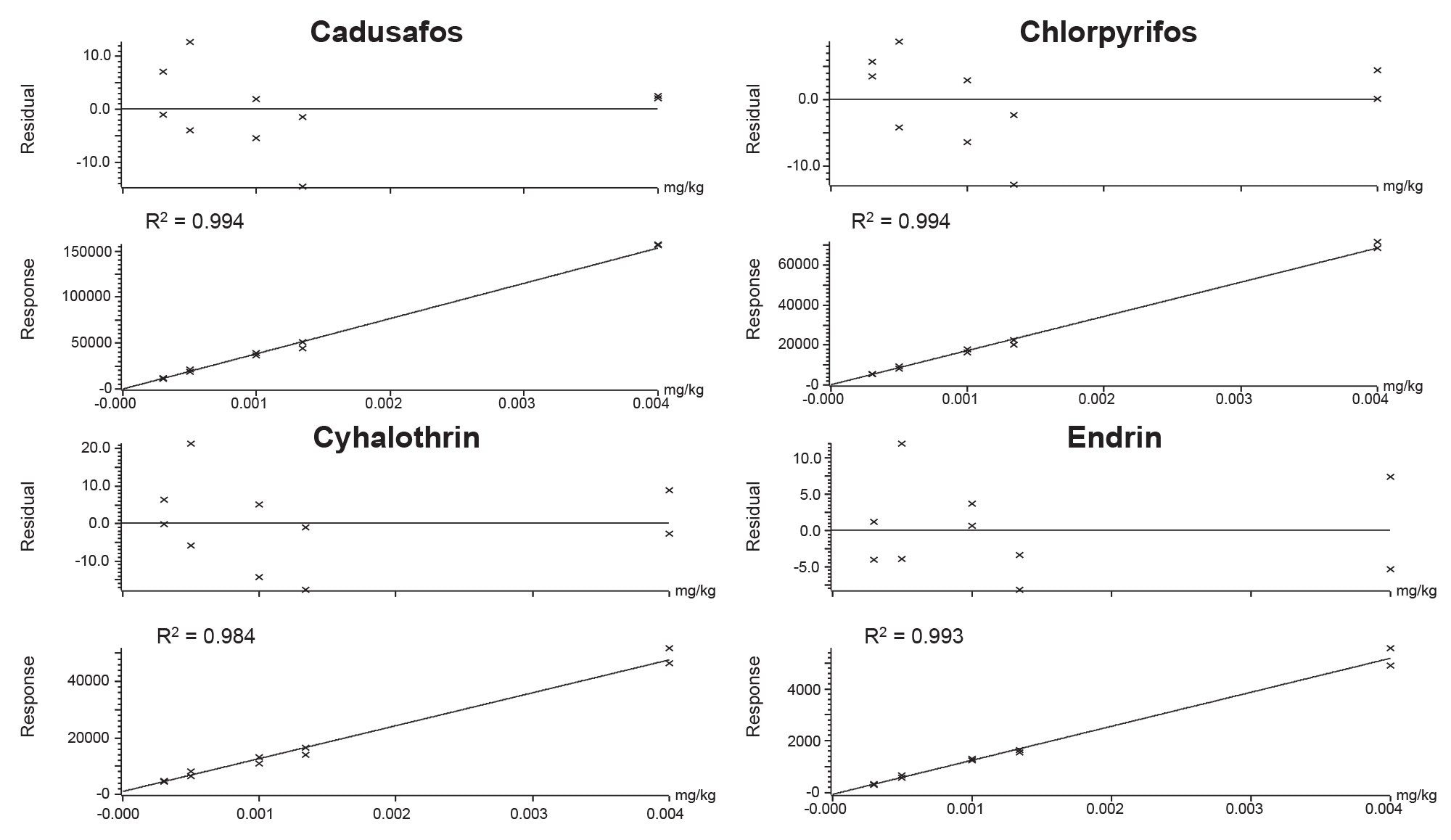
The lowest calibrated level (LCL) for each analyte was established by evaluation of the bracketed calibration graph. The performance for fluoxastrobin and tetraethyl pyrophosphate (TEPP) was considered semi-quantitative only as the calibration graph exhibited poor residuals (>20%) across the concentration range and values for coefficient of determination (r2) of <0.95. Data points at low concentrations were excluded from the calibration graph for thiometon due to poor residuals (>20%) and the value for LCL was adjusted accordingly to 0.001 mg/kg. 96% of analytes exhibited residuals well within the ±20% SANTE tolerance.6 The calibration graphs for 95% of analytes had values for r2 >0.98. Calibration graphs from the analysis of a selection of priority pesticides in baby food matrix-matched standards are given in Figure 3.
Identification criteria, retention times and ion ratios, were calculated and flagged using TargetLynx. The retention time and ion ratio of each analyte detected in each spiked sample should correspond to that of the calibration standard reference.6 The retention times of all 166 analytes were found to be within the tolerance of ±0.1 minute. The ion ratios from the analysis of the spiked samples were within ±30% of the average of calibration standards from same sequence for 94% of the analytes.
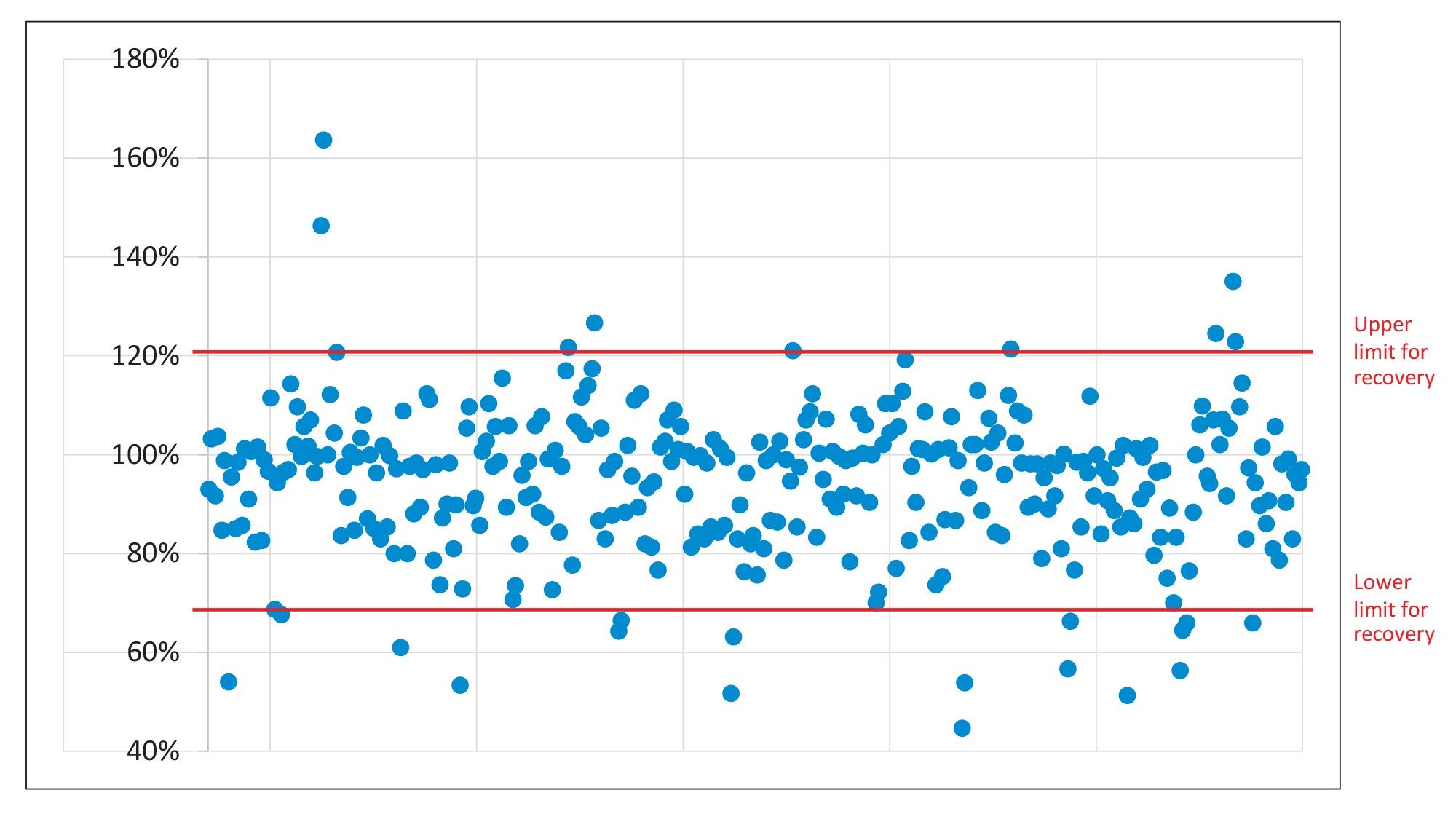
The recovery was evaluated using the data from the analysis of the six replicate spikes, at the two concentrations. The SANTE guidelines specifies an average recovery for each spike level tested to be between 70 and 120%.6 The results from analysis of the spikes at the two levels showed that 90 and 92% of the analytes were within that tolerance, respectively. Recovery of carbofuran was consistently high (>140%), presumably due to the contribution from the degradation of carbosulfan in the spikes. The remaining compounds all exhibited recoveries between 30 and 140%, but they are consistent (RSD ≤20%). A summary of the recovery results is shown in Figure 4.
The repeatability (RSDr) of the method was also satisfactory. SANTE guidelines states that RSDr for each spike level tested should be ≤20%.6 At 0.0005 mg/kg, 96% of the analytes were within this tolerance. At the higher concentration of 0.001 mg/kg, all the analytes exhibited values for RSDr ≤20%. The repeatability values are summarized in Figure 5.
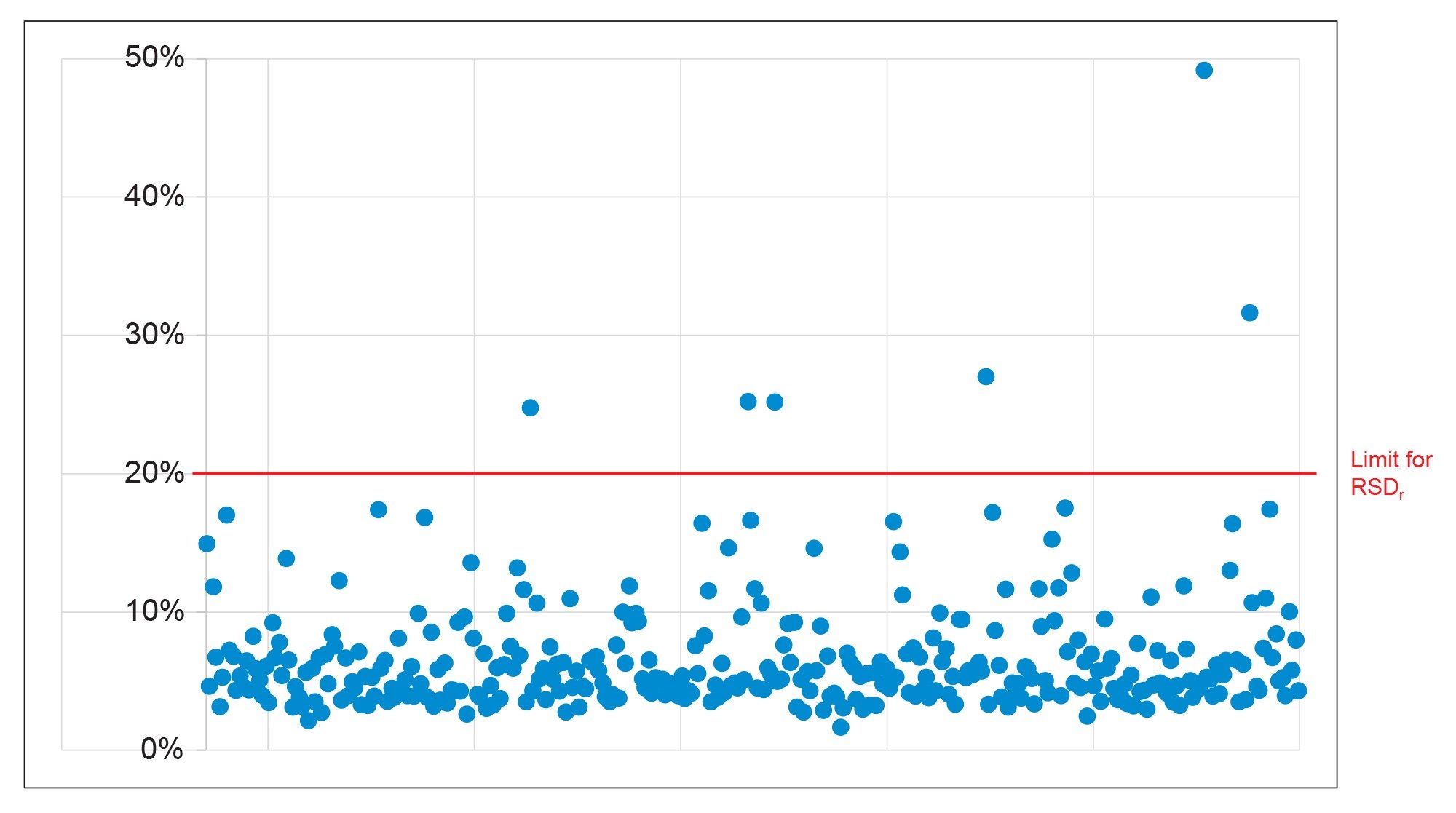
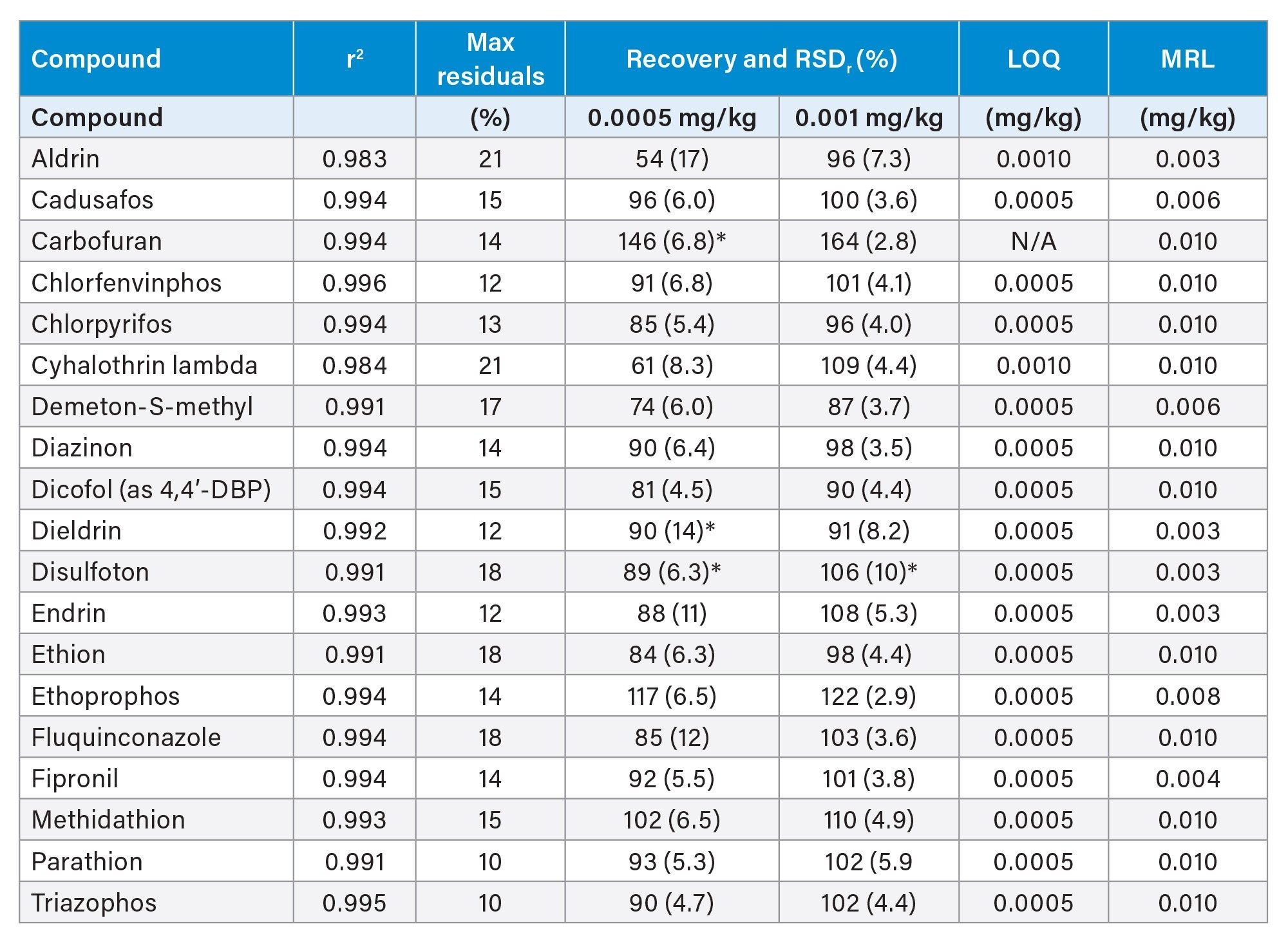
The limit of quantification (LOQ) for each compound was determined as the lowest spike level for which the above acceptance criteria were met.6 Table 1 shows a summary of the performance data for a selection of pesticides, which were considered a high priority by the EURL.
Conclusion
This application note describes a sensitive and accurate multiresidue method for the determination of pesticide residues using GC-MS/MS (Xevo TQ-XS fitted with APGC). The method allowed for reliable quantitation down to concentrations well below the MRLs specified for food for infants and young children. It was successfully validated according the SANTE guidelines, presenting results for 166 pesticides in rice-based baby food. The results from analysis of the spikes at both concentrations showed that 91% and 98% of the analytes were within the required tolerances for recovery and repeatability, respectively. The method exhibited very high sensitivity (LODs typically ≤0.0003 mg/kg) without the need for solvent exchange, PTV or large volume injection. The method is considered sensitive, specific, accurate, and suitable for the determination of residues of a wide range of GC-amenable pesticides for checking compliance with the specific MRLs set for food intended for infants and young children and also has the potential for determination at much lower concentrations.
References
- Commission Directive 2006/125/EC of 5 December 2006 on Processed Cereal-Based Foods and Baby Foods for Infants and Young Children. OJ L 339, 6.12.2006, p. 16–35.
- Commission Delegated Regulation (EU) 2021/1041 of 16 April 2021 Amending Delegated Regulation (EU) 2016/127 as Regards the Requirements on Pesticides in Infant Formula and Follow-on Formula. OJ L 225, 25.6.2021, p. 4–6.
- Cherta L et al. Application of Gas Chromatography-(Triple Quadrupole) Mass Spectrometry With Atmospheric Pressure Chemical Ionization for the Determination of Multiclass Pesticides in Fruits and Vegetables. J Chromatogr. A (2013) 1314:224–240.
- Determination of Pesticide Residues in Cucumber Using GC-MS/MS with APGC After Extraction and Clean Up Using QuEChERS. Waters Application Note 720007654, 2022.
- EURL-CF. Validation Report 38. Determination of Pesticide Residues in Rice Based Babyfood By LC-MS/MS and GC-MS/MS (QuEChERS method), 2021.
- Document No. SANTE/12682/2019. Guidance Document on Analytical Quality, Control, and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. 2019.
Annexure
720007682, August 2022