
This is an Application Brief and does not contain a detailed Experimental section.
We present a simple method for the quantification of buprenorphine and norbuprenorphine in urine. The method involves a simple SPE purification step prior to analysis by HPLC/MRM.
Buprenorphine (BUP) is a powerful semisynthetic analgesic. The molecular structure of BUP is closely related to that of morphine, yet it has been estimated to be at least 25 times more potent. Initially, it was available for the treatment of both acute and chronic pain (marketed under various trade names including; Temgesic, Buprenex, and Subutex). Following administration, BUP is absorbed and metabolised by N-dealkylation to give norbuprenorphine (norBUP).
More recently BUP has been shown to suppress heroin administration1 where the decreased physical dependence and lack of significant withdrawal symptoms make it an attractive alternative to methadone in the management of opioid addiction. However, several cases of misuse have been reported.2 Therefore it has become necessary to be able to measure BUP and norBUP in biological samples collected not only within the clinical setting (to monitor therapeutic use) but also within the penalty setting, as in the case of possible abuse.
Quantification of BUP (and the major metabolite, norBUP) in biological fluids requires a highly sensitive method since the high potency of BUP means that only very low therapeutic blood concentrations are required. Here we report a simple HPLC-MS/MS method for the determination of BUP and norBUP in urine samples.

Urine samples were prepared for HPLC-MS/MS analysis by means of a simple, generic solid-phase extraction (SPE) procedure. A Waters Oasis HLB Extraction Cartridge (1 cc/30 mg) was firstly conditioned with methanol (1 mL) followed by water (1 mL). Urine samples (500 μL, spiked with deuterated internal standards) were made up to a final volume of 1mL with water before applying to the pre-conditioned cartridge. The cartridge was then washed with 5% methanol before elution of the sample using 100% methanol (0.5 mL). Ten microlitres (10 μL) of the eluant was analysed using HPLC in conjunction with multiple reaction monitoring (MRM).
A Quattro Ultima triple quadrupole mass spectrometer fitted with Z-Spray ion interface was used for all analyses. Ionisation was achieved using electrospray in the positive ionisation mode (ES+).
HPLC analyses were performed using a Waters 2690 separations module. All aspects of system operation and data acquisition were controlled using MassLynx NT 3.5 Software with automated data processing using the MassLynx QuanLynx program.
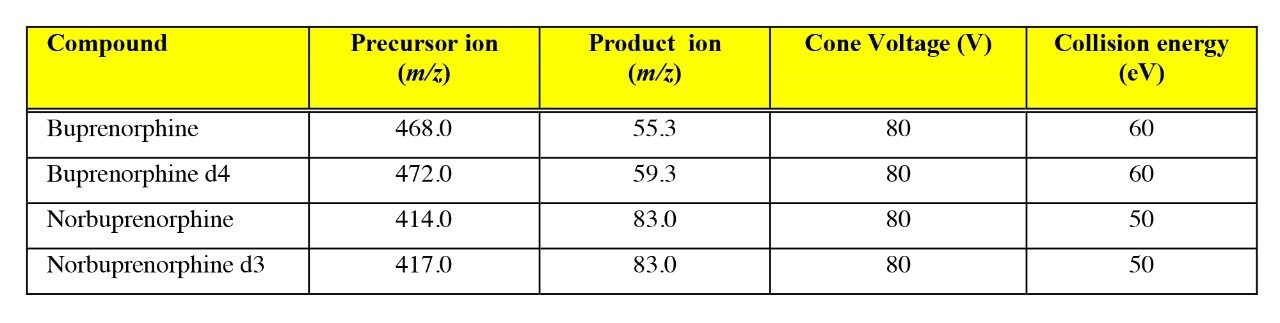
Chromatography was performed using a Waters Symmetry 300 C18 Column (2.1 x 150 mm, 5 μ) maintained at 30 °C. The column was eluted with 2 mM ammonium acetate pH 3:acetonitrile (10:90) at a flow rate of 0.25 mL/min. Details of the MRM conditions are given in Table 1.
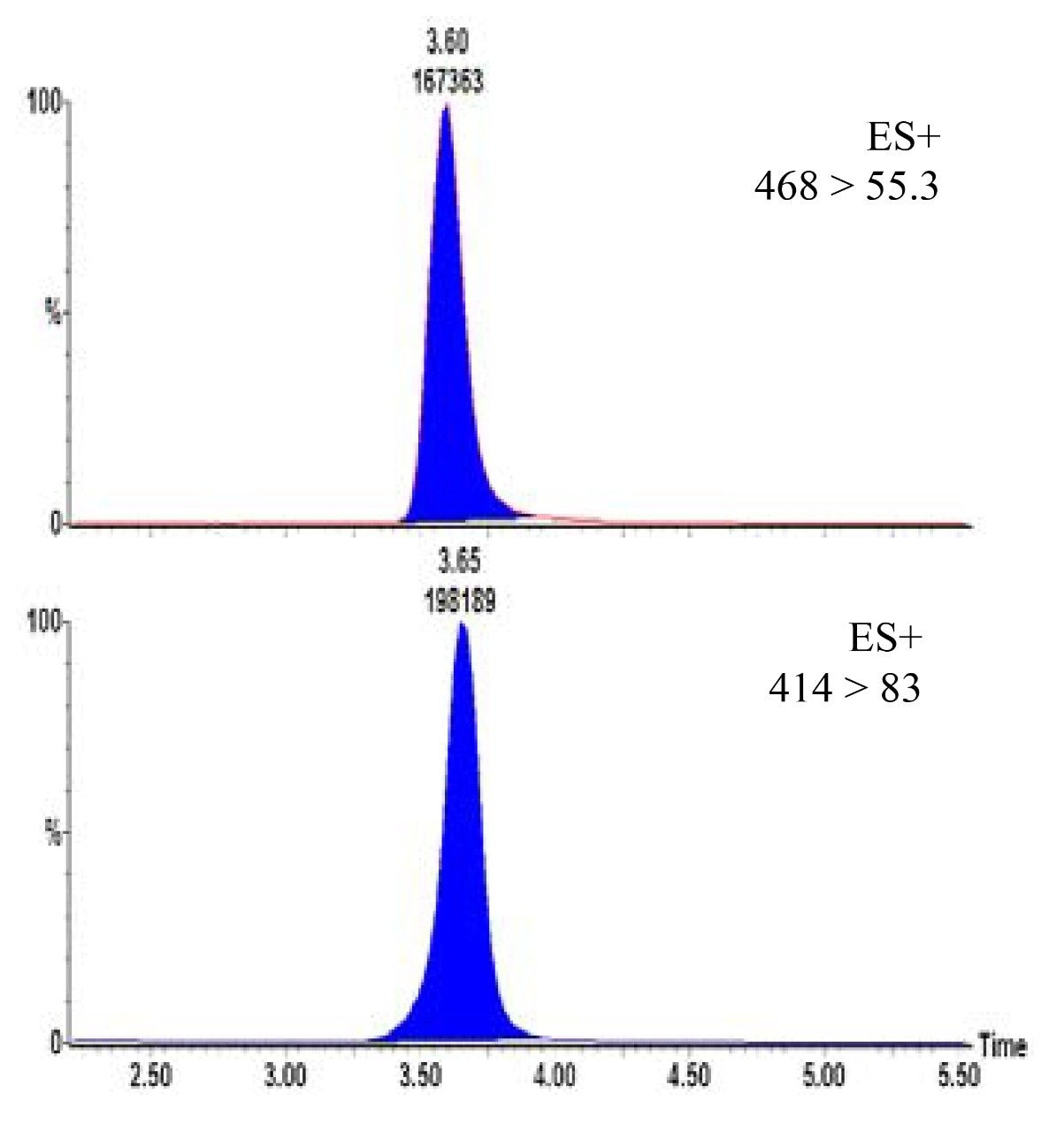
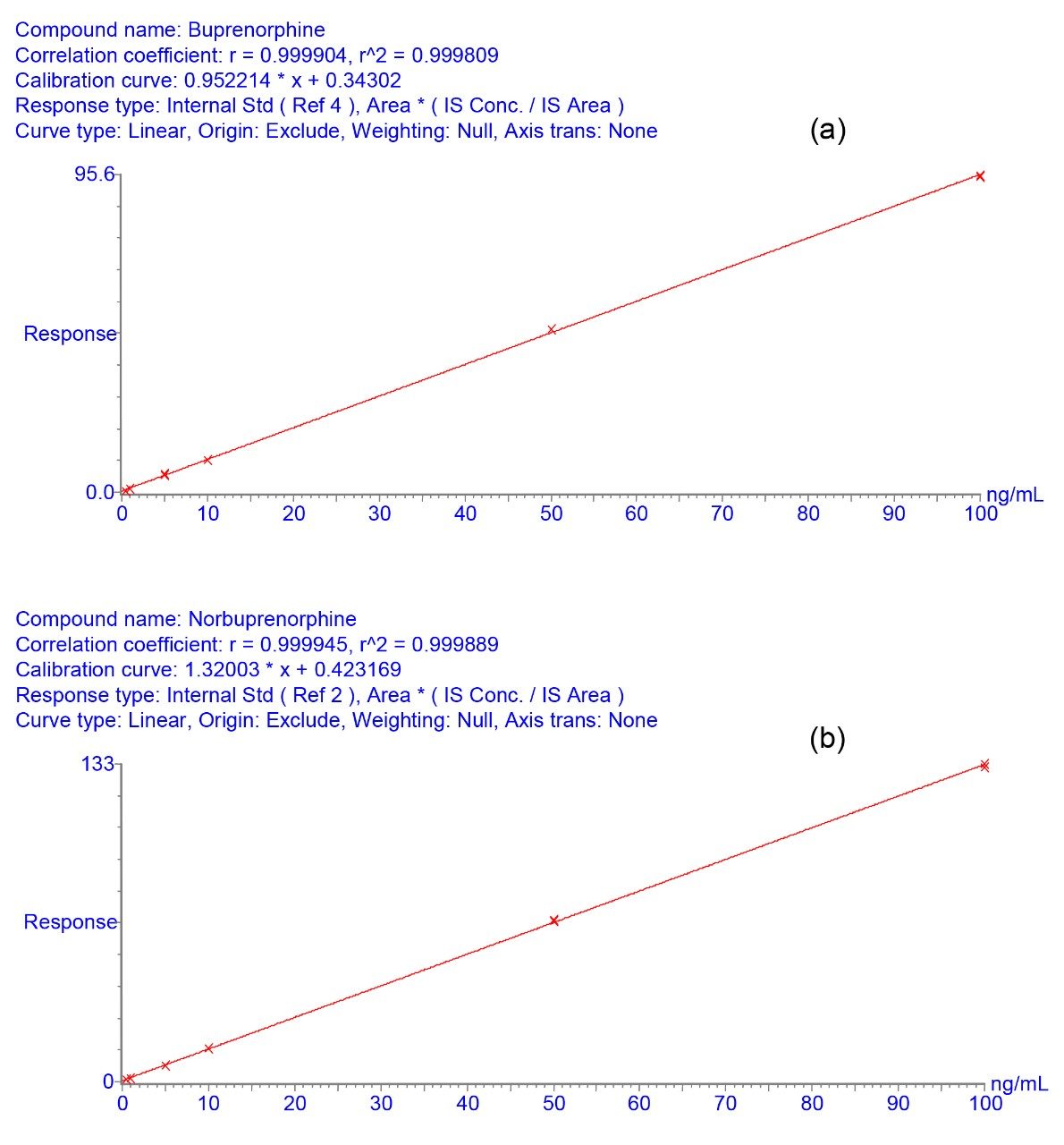
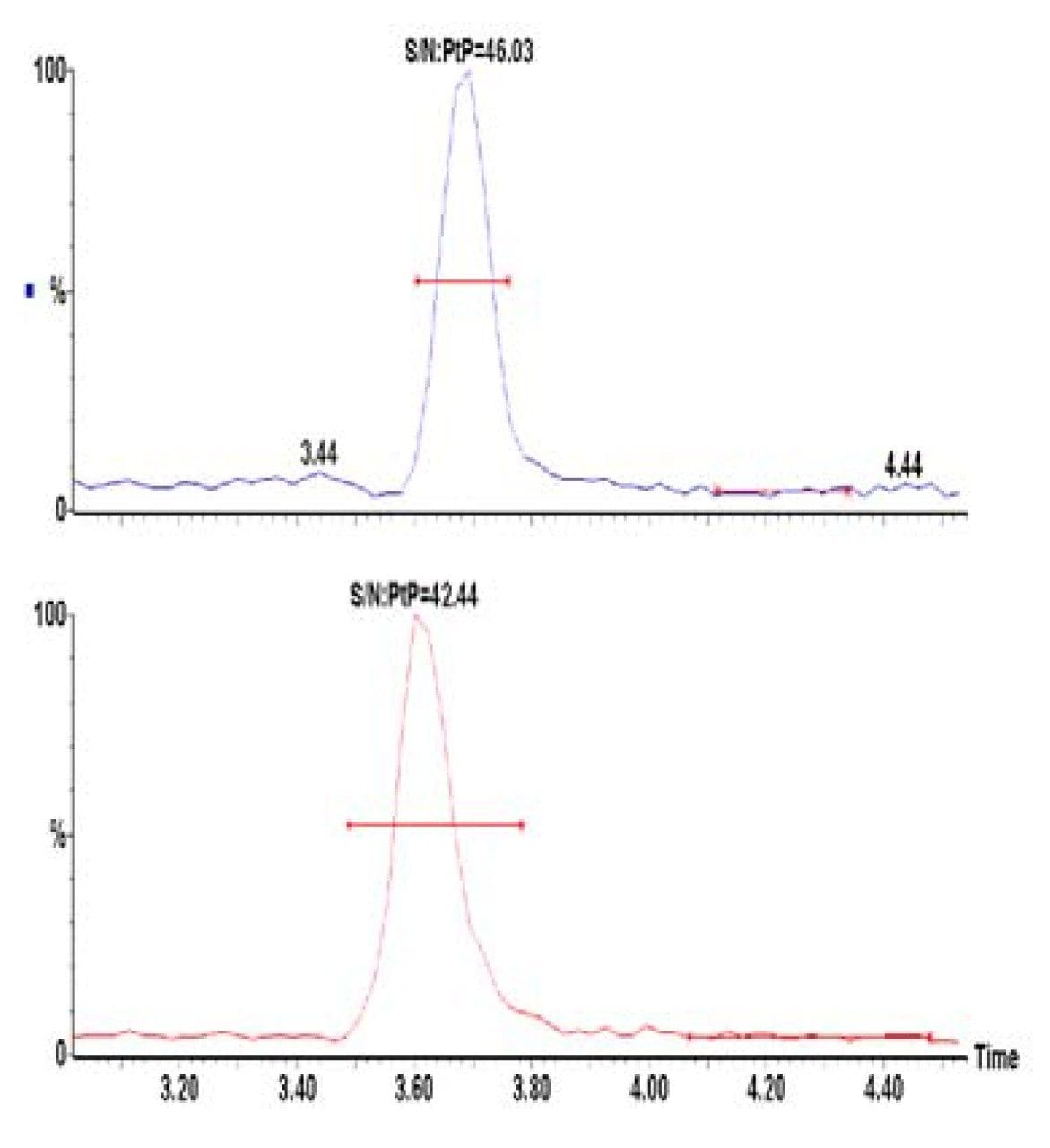
Calibrators (0.5–100 ng/mL) were prepared by adding standards to blank urine. Urine samples (either calibrators or unknown samples) were firstly extracted using the SPE method described above and then analysed (in duplicate) using HPLC-MRM. Quantification was performed by integration of the area under the specific MRM chromatogram (Figure 1). BUP and norBUP were quantified by reference to their deuterated internal standards. Typical standard curves for BUP and norBUP are given in Figures 2a and 2b respectively. Figure 3 shows the signal to noise (S:N) obtained with the 1ng/mL calibrator for both compounds. The reproducibility of analysis was assessed by replicate analysis (n=10) of the 1 ng/mL calibrator, %CV’s of 1.6 and 2.7 were obtained for BUP and norBUP respectively.
We present a simple method for the quantification of buprenorphine and norbuprenorphine in urine. The method involves a simple SPE purification step prior to analysis by HPLC/MRM.
AB44, November 2001