This is an Application Brief and does not contain a detailed Experimental section.
This document gives an overview of exact mass measurement and the benefits of routine application in both MS and MS/MS modes.
The ability to obtain exact mass measurement is a powerful tool in the identification and confirmation of compounds. It has long been recognized that if an ion can be measured with sufficient accuracy it may be possible to infer a unique elemental composition. Historically, exact mass measurement has been carried out using magnetic sector mass spectrometers, which tend to be large, expensive and require a highly trained operator to deliver good data. This has lead to the perception that exact mass measurement is difficult to achieve. The introduction of orthogonal acceleration-time of flight (oa-TOF) mass spectrometry has greatly simplified exact mass measurement and provided more affordable and compact benchtop instrumentation. Many scientists given the choice of exact mass or MS/MS capabilities would choose MS/MS without appreciating what exact mass can offer. This document gives an overview of exact mass measurement and the benefits of routine application in both MS and MS/MS modes.
With the exception of carbon (against which the rest are measured), the elements in the periodic table do not have integer masses (for example, H = 1.0078, C = 12.0000, N = 14.0031 and O = 15.9949 amu). Therefore it is possible to have different combinations of atoms that have the same nominal mass but different exact masses. The classic, simple examples are CO = 27.9949, N2 = 28.0061 and C2H4 = 28.0313. If a mass spectrometer that measures only nominal mass (or to one decimal place), such as a quadrupole or ion trap, were used then these three molecules would appear identical. If an instrument were used that can measure mass accurately to the third or fourth decimal place then the three spectra would be different. This becomes important to people using organic mass spectrometry because if the elemental compositions of two organic molecules differ by just substituting one of these elemental combinations for another, the molecular weights of the two compounds can only be distinguished by an exact mass measurement.
The generation of exact mass data using an oa-TOF is routine. Although these instruments are very stable, it is good scientific practice to use a reference compound as a lock mass (see glossary of terms) to ensure good mass measurement. Waters Micromass oa-TOF instruments can be equipped with a reference sprayer inlet (LockSpray) that allows a known solution to be introduced into the API source through a separate line that is sampled independently by the instrument. A single reference mass peak can then be used to automatically correct each spectrum generated, ensuring mass accuracy to better than 5 ppm. This occurs in real time acquisition and is transparent to the analyst. Aside from the advantage of mass measurement accuracy, oa-TOFs have an efficient duty cycle giving high sensitivity, enhanced mass resolution (5000 - 17,500 FWHM) and rapid acquisition rates (up to 10 spectra per second).
|
Accurate mass |
For mass spectrometry, the term accurate is generally used to describe exact mass data that lies within 5 ppm of the calculated mass |
|
Exact mass |
Measurement of a mass or mass-to-charge ratio to 3 or 4 decimal places |
|
Isobaric |
Having the same mass |
|
Nominal mass |
Mass measured (or quoted) to integer accuracy |
|
Error |
Deviation of the measured mass from the true mass usually quoted in ppm or milliDaltons (mDa) |
|
Parts-per-million (ppm) |
Measurement of mass accuracy defined by ΔM / M x 106 (where ΔM is the mass error) this varies with mass for the same absolute error |
|
u |
Unified atomic mass unit - this is the basic unit of measurement of atomic mass equivalent to 1/12 of the mass of a 12C atom |
|
Dalton (Da) |
Equivalent to a unified atomic mass unit (amu). mDa is one thousandth of a Da |
|
amu |
Atomic mass unit equivalent to 1/16 of the mass of a 16O atom. This is no longer the accepted unit of atomic mass but is very often used mistakenly in place of u. |
|
Lock mass |
A peak in a mass spectrum of known (exact) mass that can be used to correct the mass assignment of the spectrum |
|
Internal standard |
A compound, of known composition, introduced into the mass spectrometer that produces a mass peak for use as a lock mass |
|
Mass resolution |
A measure of the width of a peak relative to its mass can be defined by valley or peak width at half height |
|
Full width half maximum (FWHM) |
Definition of resolution based on peak width M / ΔM (where ΔM is peak width at half its maximum height) for the same absolute width resolution varies with mass |
An exact mass measurement can help to determine the elemental composition of a compound but it does not give information about the structure. If you can measure the mass of an ion with sufficient accuracy then it is feasible to determine a unique elemental composition for that ion. For structural elucidation of complete unknowns exact mass spectrometry in conjunction with NMR is a very powerful tool. It is often assumed that MS/MS can be used to determine the structure of an unknown. While this is sometimes true for compounds that give predictable fragmentation patterns, such as peptides, it is rarely the case for low molecular weight compounds.
For a given mass accuracy, the number of possible elemental compositions increases with increase in the measured mass and it may not be possible to infer a unique formula. However there is other evidence that can be used to limit the number of possibilities.
The operation of an oa-TOF is different to that of a quadrupole or an ion trap but, after familiarization, exact mass measurement is a routine operation. Lock mass correction and generation of exact mass spectra can be accomplished automatically in real time.
Early oa-TOF instruments with time-to-digital converters (TDC) had linear ranges limited to 2 -2.5 orders of magnitude. Waters systems employ a patented correction algorithm that extends the linear range. This automatic correction allows exact mass measurement and quantification over a range greater than 3 orders of magnitude. Recent advances in ion optics have allowed the latest instruments to achieve up to 4 orders of linear range.
For purely quantitative applications a quadrupole instrument operating in SIR or MRM mode would be the instrument of choice due to offering better sensitivity and wider dynamic range. However, an oa-TOF has certain advantages for quantification.
(i) An oa-TOF always generates full mass spectra giving both qualitative and quantitative data from the same analysis. (ii) All masses are acquired simultaneously during an acquisition so that any number of analytes can be quantified in the same run and data can be re-processed at a later date to look for compounds that weren't previously of interest. (iii) Specific 'exact mass' chromatograms can be plotted to reduce background chemical noise and improve detection levels.
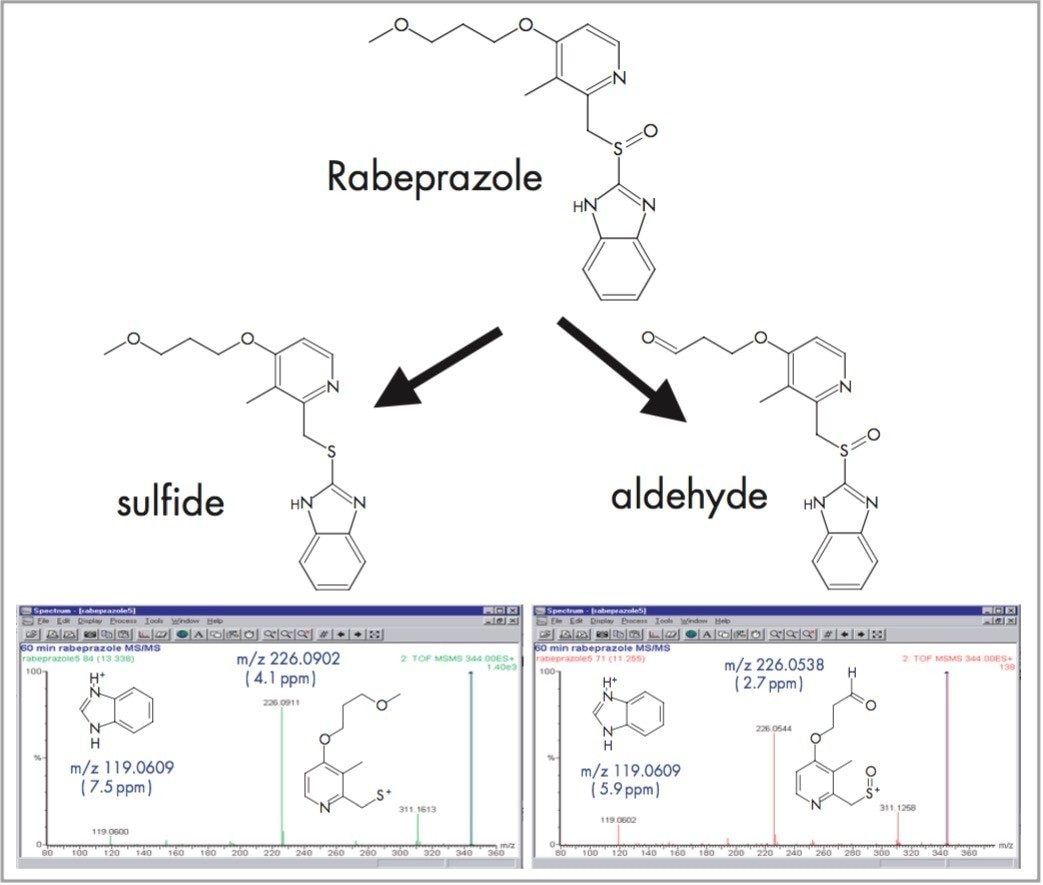
The in vitro metabolism of Rabeprazole with rat hepatocytes generates a number of metabolites. Two of these, the aldehyde and the sulfide, produce an [M+H]+ ion with the same nominal mass (m/z 344) but which are actually separated in mass by only 36mDa. At this mass, 5 ppm accuracy gives a maximum error of only 1.7 mDa, so it is easy to distinguish these 2 metabolites using oa-TOF. If a quadrupole or ion trap mass spectrometer were being used the analyst would attempt to use MS/MS to differentiate these compounds. But, in this case, the 2 metabolites generate fragment ions of the same nominal mass and the spectra look very similar. Only an exact mass fragment ion spectrum can show that the fragment ions at m/z 226 are different in the 2 spectra (Figure 1). Nominal mass measurement and MS/MS would not have been able to distinguish these two metabolites.
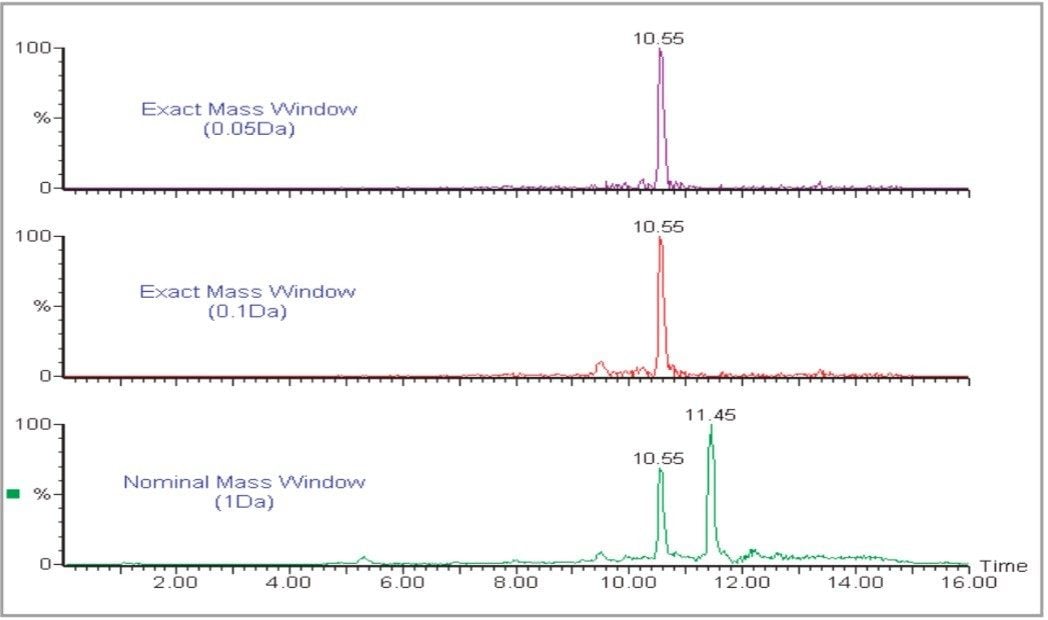
Grape extracts (ethylacetate extraction) were analyzed using LC-MS on a Waters Micromass LCT equipped with a LockSpray interface. Exact mass data were acquired at a rate of 1 spectrum/second and mass chromatograms for the [M+H]+ ions of each of 17 pesticides were plotted and integrated to allow quantification of the pesticides against a calibration curve produced from matrix-matched standards. Grape extracts produce a complex matrix containing many components and, if a nominal mass chromatogram (1 amu window) is used for integration, there is a high potential for interference. Due to the resolution and exact mass capability of oa-TOF it is possible to apply a very narrow mass window to the extracted chromatograms. These exact mass chromatograms are more specific and allow nominally isobaric interferences to be filtered out. This has the effect of reducing the background noise level without reducing the analyte signal, thereby improving signal to noise and reducing detection levels. An example is shown in Figure 2 for carbosulfan in grape extracts.
720000764, October 2003