Antibody drug conjugates (ADCs) represent a rapidly growing class of biotherapeutic drugs for the treatment of cancer. ADCs offer the selectivity of an therapeutic antibody with the potency of a cytotoxic agent such as a synthetic drug. ADC design, in part, relies on predictable conjugation chemistry that preserves antibody binding activity while facilitating reproducible characteristics that can be used as metrics for assessing critical quality attributes (CQAs) to ensure a safe and effective ADC product. Interest from pharmaceutical companies to deliver ADC biotherapeutics to market is intensifying.
To support this, characterization methods that are efficient and adaptable to evaluate CQAs associated with this novel class of biotherapeutic drug are needed.
Empower 3 Software represents one of Waters’ solutions to challenges in understanding ADCs. This chromatographic data system can be used to streamline the analytical workflow through an integrated approach to data acquisition, processing, and reporting of experimental results. As one part of its integrated informatics toolset, Empower's custom field calculations are seamlessly incorporated into the ADC data analysis workflow to help analysts readily determine drug-to-antibody ratio (DAR) that corresponds to drug load distribution.
In this application note, learn about Waters' end-to-end and automatable workflow that takes advantage of our integrated UPLC/QTof-MS analytical technologies. We demonstrate that, by using our efficient method deployment approach, this workflow can help you maintain high productivity in ADC characterization.
Learn more about Waters' solutions for ADC characterization.
Antibody drug conjugates (ADCs) represent a rapidly growing class of biotherapeutic drugs for the treatment of cancer.1 ADCs offer the selectivity of an antibody with the potency of a cytotoxic agent such as a synthetic drug.2 ADC design, in part, relies on predictable conjugation chemistry that preserves antibody binding activity (Figure 1A) while facilitating reproducible characteristics that can be used as metrics for assessing critical quality attributes (CQAs) to ensure a safe and effective ADC product.3

Cysteine-conjugated ADCs are a sub-class of biotherapeutic drugs that are manufactured with well-known conjugation chemistry. Drug payloads are attached through a linker to monoclonal antibodies (mAbs) via the thiol groups. Thiols are generated by the reduction of inter-chain disulfide bonds, and expected drug load occurs in intervals of 2, 4, 6, and 8 as shown in Figure 1B due to the formation of two thiols from each disulfide.
The Protein-Pak Hi Res HIC column (4.6 x 100 mm, 2.5 μm, p/n: 186007583) was conditioned prior to use according to its care and use manual. Chemical reagents were purchased from Sigma-Aldrich and used as received. A cysteine-conjugated ADC was prepared by a collaborator at a concentration of 10 mg/mL in formulation buffer. Samples were prepared for analysis at a concentration of 2 mg/mL in 1M ammonium sulfate ((NH4)2SO4).
|
LC system: |
ACQUITY UPLC H-Class |
|
Detector: |
ACQUITY UPLC TUV |
|
Absorption Wavelength: |
280 nm |
|
Vials: |
Total Recovery vial: 12x32 mm glass, screw neck, cap, nonslit |
|
Column: |
Protein-Pak Hi Res HIC, 4.6 x 100 mm, 2.5 μm |
|
Column temp.: |
25 °C |
|
Sample temp.: |
4 °C |
|
Injection vol.: |
10 μL |
|
Mobile phase A: |
125 mM Phosphate buffer, pH 6.7 with 2.5 M (NH4)2SO4 |
|
Mobile phase B: |
125 mM phosphate buffer, pH 6.7 |
|
Mobile phase C: |
Isopropyl alcohol |
|
Mobile phase D: |
Water |
|
Data Management: |
Empower 3 Software, SR1, FR2 |
|
Time (min) |
Flow rate (mL/min) |
%A |
%B |
%C |
%D |
|---|---|---|---|---|---|
|
Initial |
0.7 |
50.0 |
0.0 |
5.0 |
45.0 |
|
10.0 |
0.7 |
0.0 |
50.0 |
5.0 |
45.0 |
|
15.0 |
0.7 |
0.0 |
50.0 |
5.0 |
45.0 |
|
15.01 |
0.7 |
50.0 |
0.0 |
5.0 |
45.0 |
|
30.0 |
0.7 |
50.0 |
0.0 |
5.0 |
45.0 |
The conjugation process, which is dependent on the reactant concentrations, can result in variable drug-to-antibody-ratio (DAR, Figure 2) and subsequently impact the efficacy and safety of the ADC. Therefore monitoring the drug distribution and drug load of ADCs during the manufacturing process is of critical importance for pharmaceutical companies.
In the absence of a dedicated workflow, analysts will often perform manual calculations based on hydrophobic interaction chromatography (HIC) peak area for the determination of DAR based on drug load distribution. Waters’ chromatographic data system (CDS), Empower 3 Software, can be used to streamline the analysis of ADCs through an integrated approach to data acquisition, processing, and reporting of experimental results. This process, which can be fully automated, makes the Empower CDS ideal for increasing productivity through efficient method deployment in the characterization of ADCs.
The objective of this application note is to demonstrate the ability to automatically determine DAR values corresponding to the drug load distribution of cysteine-conjugated ADCs using Empower 3 Chromatography Data Software. A cysteine-conjugated ADC was used as a model conjugate to test the application.
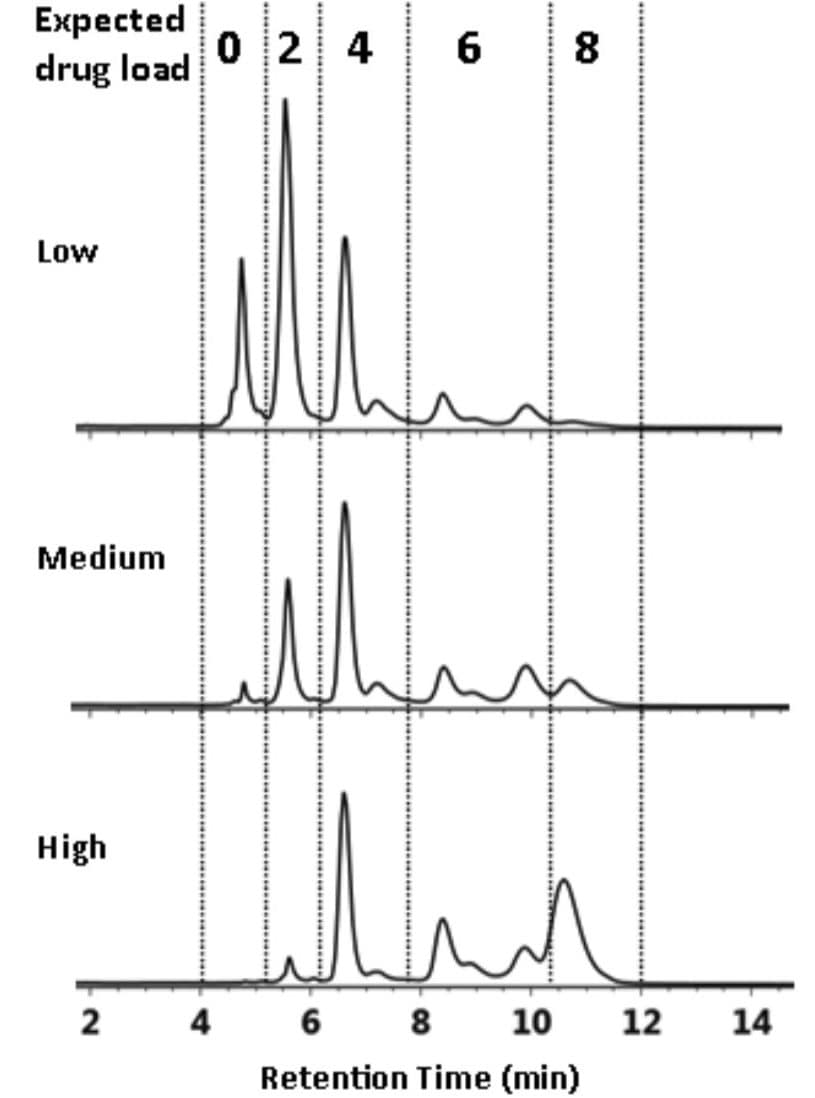
Chromatograms of separations of cysteine-conjugated ADCs using HIC are often comprised of multiple peaks where groups of peaks represent positional isomers of a conjugated antibody (Figure 3A).3
With its high-fidelity separations, the ACQUITY UPLC H-Class System delivers reproducible analyses; the researcher can then take advantage of informatics tools in Empower 3 Software to accurately group peaks by retention time for calculation of the DAR value based on drug distribution. This results in a highly efficient approach to method development during characterization.
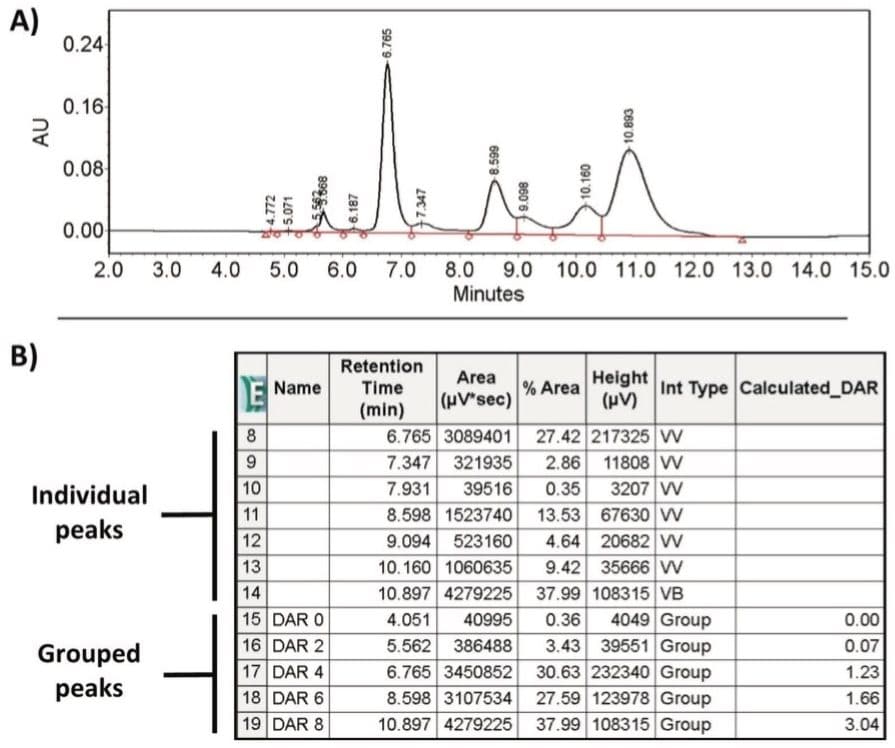
As shown in Figure 3A, the multiple components associated with individual drug loads (DAR 0 through DAR 8) are entered into the Timed Groups tab (Figure 3B) of the processing method based on expected retention times. The ability to manage identification of components in separations like this makes Empower 3 Software ideal for characterizing challenging biotherapeutics such as ADCs.
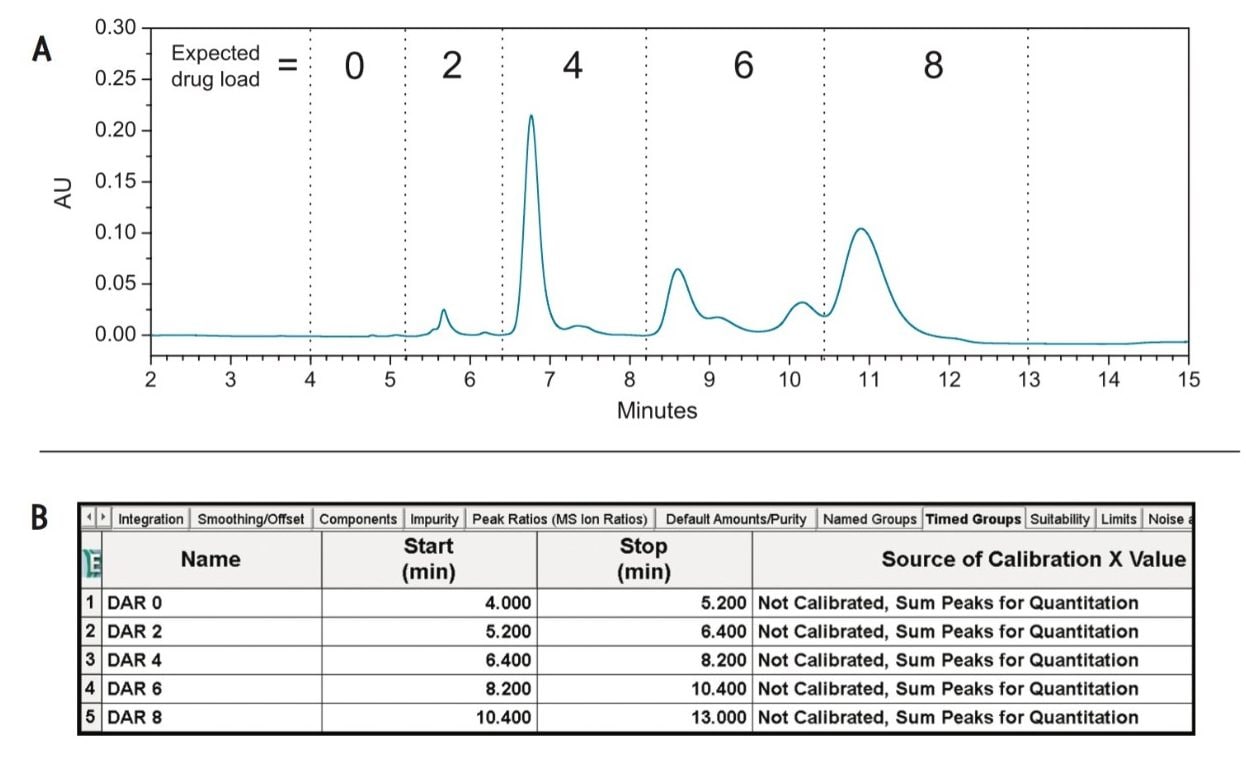
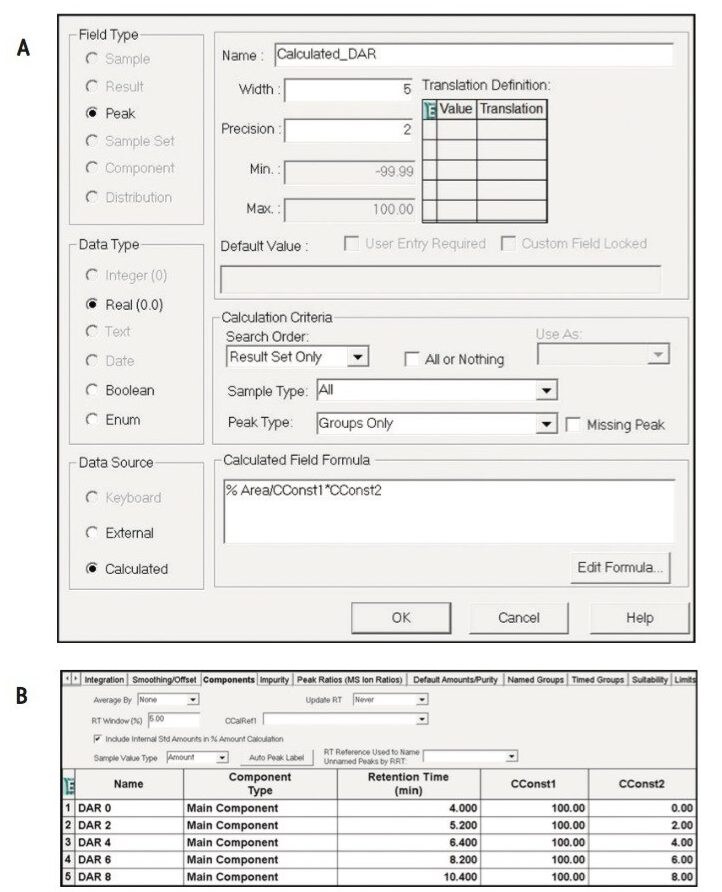
Empower 3 Software provides a myriad of informatics tools – which are designed to increase productivity in data analysis – that can be deployed for the automation of calculations associated with CQAs of cysteine-conjugated ADCs such as DAR values. This is achieved through the use of the Custom Fields, which are generated in the project properties from the system configuration manager as shown in Figure 4A.
Efficient processing of results using Empower is, in part, made possible through the ability to set criteria for custom field calculations. As shown in Figure 4A, by selecting Groups Only in the Peak Type window, the custom calculation shown in the formula window will only be applied to the grouped data as defined in Figure 3B. Furthermore, Empower’s flexibility to use numerical constants from the Component Manager (Figure 4B) facilitates the use of custom calculations such as DAR values for cysteine-conjugated ADCs.
The custom fields, upon initial set-up, can be incorporated into the analysis workflow. To demonstrate this, 20 µg of a cysteine-conjugated ADC sample at a concentration of 2 mg/mL in 1M (NH4)2SO4 was injected on a Protein-Pak Hi Res HIC column (4.6 x 100 mm, 2.5 μm) and separated using a 10-minute gradient as shown in Figure 5A. As shown in Figure 5B, Empower automatically reports the retention time and associated peak area for the individual peaks as well as the grouped peaks as defined in Figure 3B.
As part of the processing method, Empower automatically calculates and displays the individual DAR values based on drug distribution (DAR 0 = 0.00, DAR 2 = 0.08, DAR 4 = 1.32, DAR 6 = 1.70, DAR 8 = 2.75) for the cysteine-conjugated ADC sample using the custom field calculation defined in Figure 4A. This process, which automates the calculation of CQAs, demonstrates that the Empower 3 Software is well-suited for increasing productivity in the characterization of antibody drug conjugates.
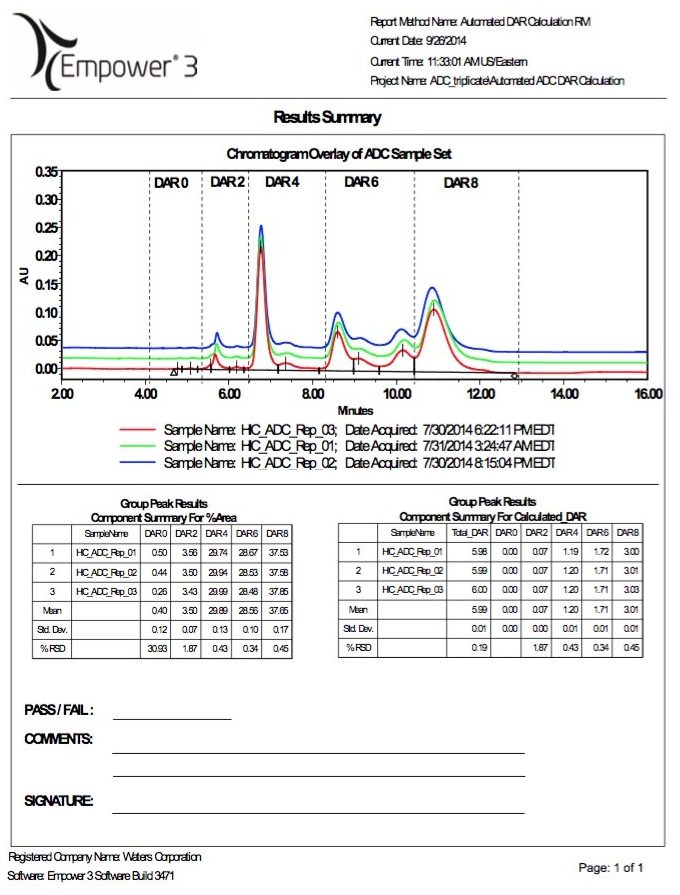
Empower 3 Software additionally features powerful reporting functionality that is designed to provide researchers with meaningful analytical data and summaries for management review. Report templates can be readily constructed and customized for assessment of results.
An example of a report template designed for reporting the DAR and drug distribution for cysteine-conjugated ADC characterization is shown in Figure 6. Using the results from Figure 5B, a summary report of the relative area and corresponding individual and total DAR values based on drug distribution along with the corresponding statistical results (e.g., mean and % RSD) is generated after data acquisition and processing.
Offering the flexibility of custom reporting templates that can be designed to meet a laboratory’s specific analytical and communication needs, Empower 3 Software is a powerful partner to an ACQUITY UPLC H-Class System. Together they provide an integrated method development approach for the acquisition, processing, and reporting of results in the characterization of cysteine-conjugated ADCs.
With a modality that has the potential to redefine cancer treatment, interest from pharmaceutical companies to deliver ADC biotherapeutics to market is intensifying. To support this, characterization methods that are efficient and adaptable to evaluate novel CQAs associated with this new class of biotherapeutic drug are needed.
Empower 3 Software represents one of Waters’ solutions to these challenging problems. This chromatographic data system can be used to streamline the analytical workflow through an integrated approach to data acquisition, processing, and reporting of experimental results. As one part of its integrated informatics toolset, custom field calculations are seamlessly incorporated into the ADC data analysis workflow to address processing challenges facing today’s analysts.
This end-to-end automatable workflow illustrates that Waters’ integrated analytical technology solutions are ideal for increasing productivity through efficient method deployment in the characterization of antibody drug conjugates.
720005192, September 2014