This application note demonstrates the separation of Canagliflozin and its isomeric impurities from its other impurities.
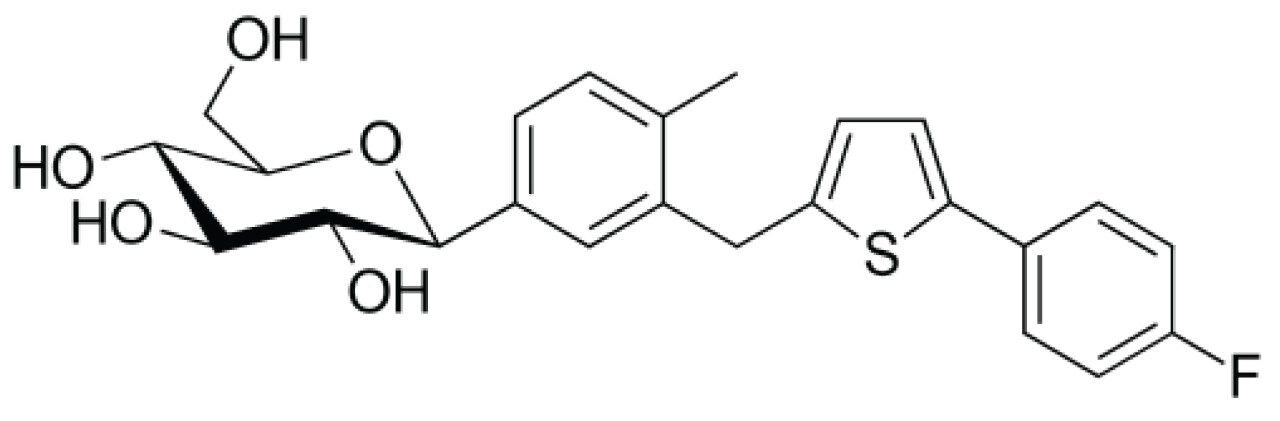
Canagliflozin is an anti-diabetic beta-isomeric drug used to improve glycemic control in people with type 2 diabetes. In extensive clinical trials, canagliflozin produced a consistent dose-dependent decrease in HbA1c levels to 0.77% to 1.16% from initial HbA1c levels of 7.8% to 8.1% when administered either as monotherapy or in combination with metformin, sulfonylurea, pioglitazone, or in combination with insulin. When added to metformin, canagliflozin 100 mg daily was shown to be non-inferior to both sitagliptin 100 mg daily and glimepiride in reducing HbA1c levels at one year, whilst canagliflozin 300 mg successfully demonstrated statistical superiority over both sitagliptin and glimiperide in decreasing HbA1c levels. Secondary efficacy endpoints like higher reductions in weight and blood pressure (versus sitagliptin and glimiperide) were also observed in studies. Canagliflozin produces beneficial effects on HDL cholesterol whilst increasing LDL cholesterol to produce no change in total cholesterol.
In development of generic drug for ANDA approval, synthesis of Canagliflozin often starts with the starting materials containing sugar moieties having alpha and beta isomers in equimolar concentrations. These impurities of starting material serve as a potential source of undesired isomeric impurities in the final API, which must be separated and isolated for their structural information and enable faster turnaround time of analysis which are essential for generic ANDA submission. It is quite challenging to separate alpha and beta isomers in normal and reverse phase chromatography and hence it is difficult to purify these isomers.
UltraPerformance Convergence Chromatography (UPC2) is a separation technique that leverages the unique properties of CO2 at or near its supercritical state. When mixed with organic solvents, ACQUITY UPC2 provides a higher separation efficiency, speed, and selectivity needed for a complex sample matrix which otherwise would be difficult to separate in normal or reversed-phase (RP) chromatography. This application note demonstrates the separation of Canagliflozin and its isomeric impurities from its other impurities in shorter runtime and thus enabling lesser consumption of organic solvent rendering true meaning to the “Go Green” tagline of convergence chromatography.
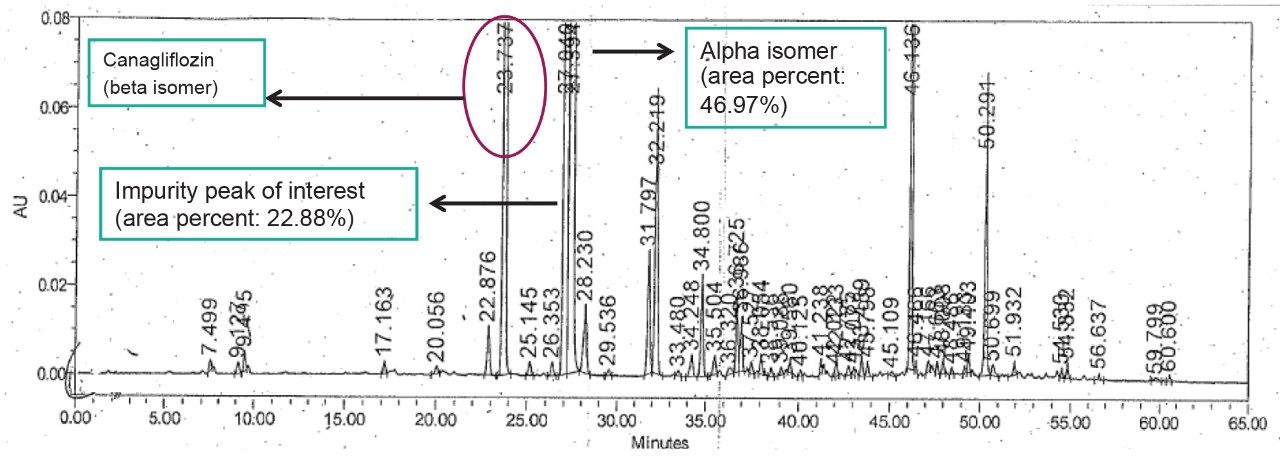
The Canagliflozin and its isomeric impurities were being analyzed by RP chromatography. Reversed-phase chromatography analysis time is about 65 minutes and does not provide enough resolution for the targeted isomeric entities. The challenge with this separation in reserved-phase chromatography was scale-up for Prep system. ACQUITY UPC2 was effectively used for screening methods to achieve the desired resolution between the isomeric peaks and for scale up to Prep SFC systems. Furthermore, with increased column loading the ACQIUTY UPC2 system can be used for analysis and accurate assay of the isomeric impurities as well.
|
System: |
ACQUITY UPC2 with ACQUITY UPC2 PDA Detector |
|
Software: |
Empower 3 |
|
Detection: |
UV at 290 nm from UV range 200–430 nm (compensation reference 330–430 nm) |
|
Column: |
ACQUITY UPC2 Trefoil AMY1 2.5 μm, 3 mm x 150 mm (P/N 186007460) |
|
Column temp.: |
45° C |
|
Sample temp.: |
10° C |
|
Sample concentration: |
250 ppm |
|
Injection volume: |
5 μL |
|
Flow rate: |
1.5 mL/min |
|
Mobile phase A: |
Compressed CO2 |
|
Mobile phase B: |
0.1% Trifluoroacetic acid in methanol: isopropyl alcohol (50:50) |
|
Run time: |
11 minutes |
|
ABPR pressure: |
2000 psi |
|
Gradient: |
5% B for 0.8 minute, ramp to 50% of B in 7 minutes, hold at 50% B for 1 minute, and return to 5% B in 0.5 minutes to equilibrate up to 11 minutes |
|
Diluent: |
Acetonitrile |
|
System: |
ACQUITY SQ Detector |
|
Software: |
Empower 3 |
|
Cone voltage: |
30 V |
|
Capillary voltage: |
3 KV |
|
Desolvation temp.: |
300 °C |
|
Desolvation gas: |
550 L/Hr |
|
Cone gas: |
20 L/Hr |
|
Make up solvent: |
Methanol with 0.1% Acetic acid |
|
Make up solvent flow: |
0.3 mL/min |
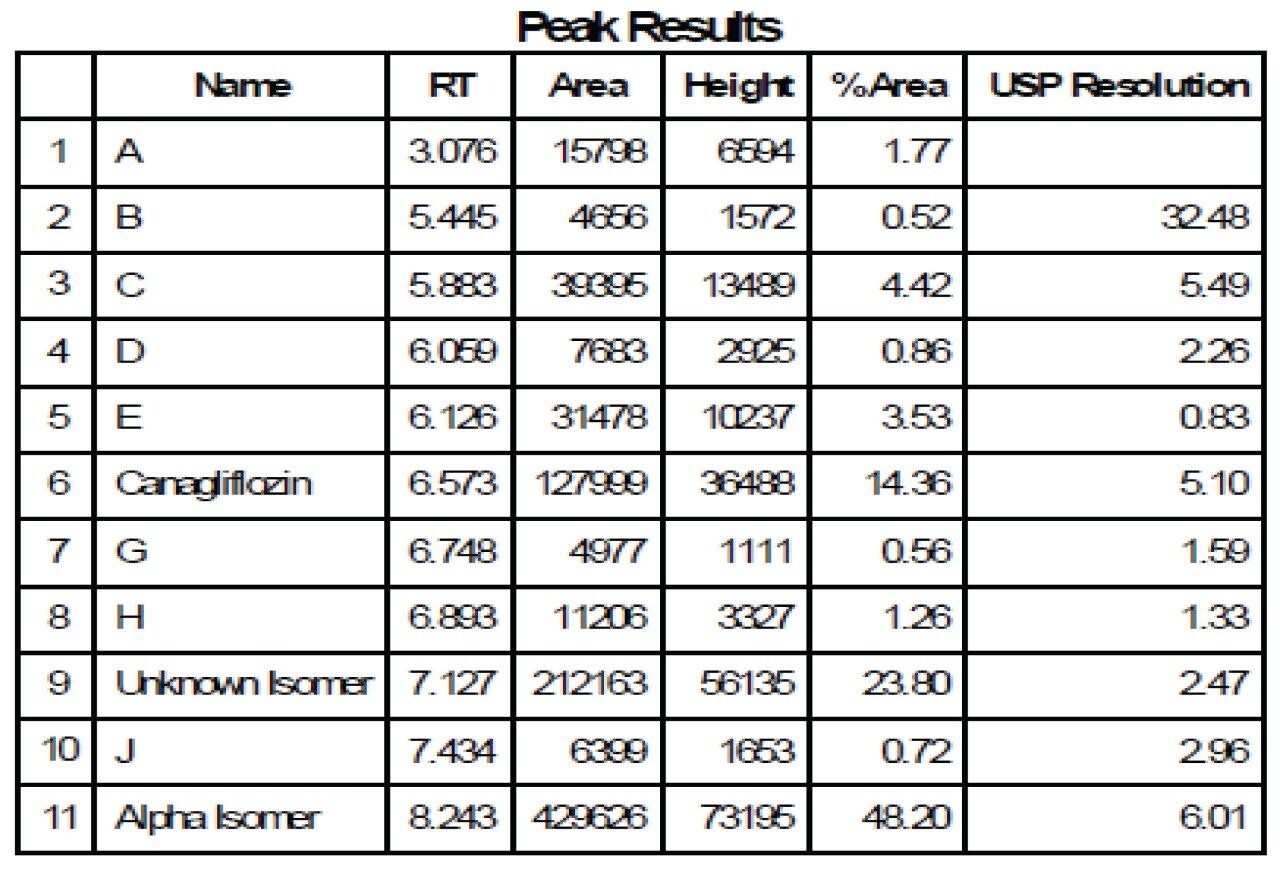
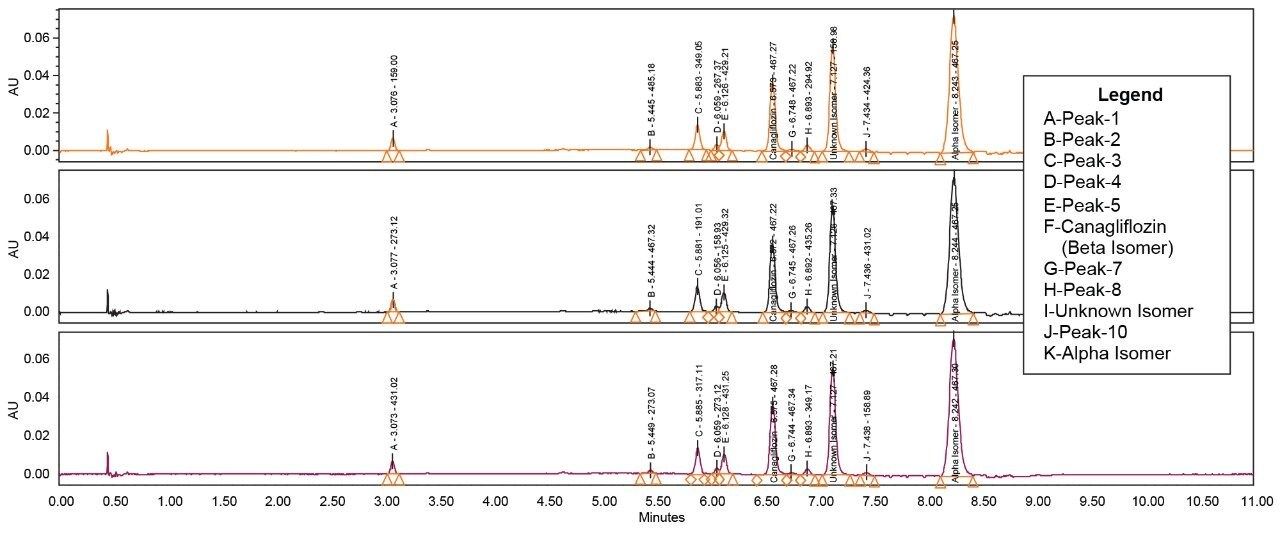
The API and its isomeric impurities were separated and identified based on the area percent of the peaks observed in UV and corresponding MS spectral data generated orthogonally with ACQUITY SQ Detector (Figures 3, 4, 5, and 6). The retention time of beta, unknown isomeric impurities, and alpha were 6.5 min, 7.1 min, and 8.2 min respectively. USP resolution of all the desired compounds was within acceptable range (5.10, 2.47, and 6.01 for Canagliflozin beta isomer, unknown isomer, and alpha isomer respectively) [Table 1].
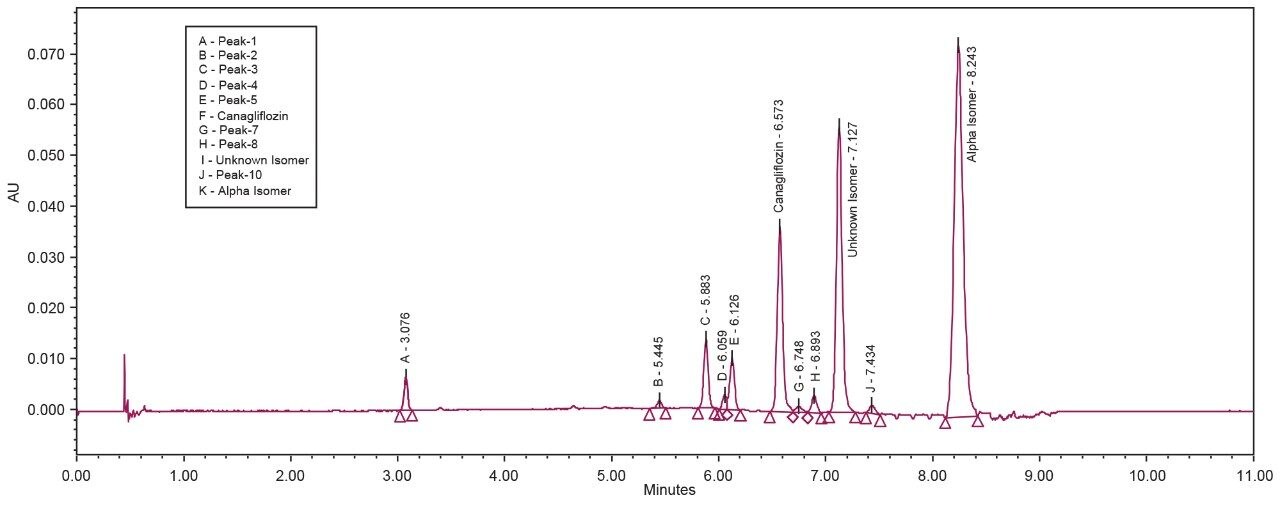
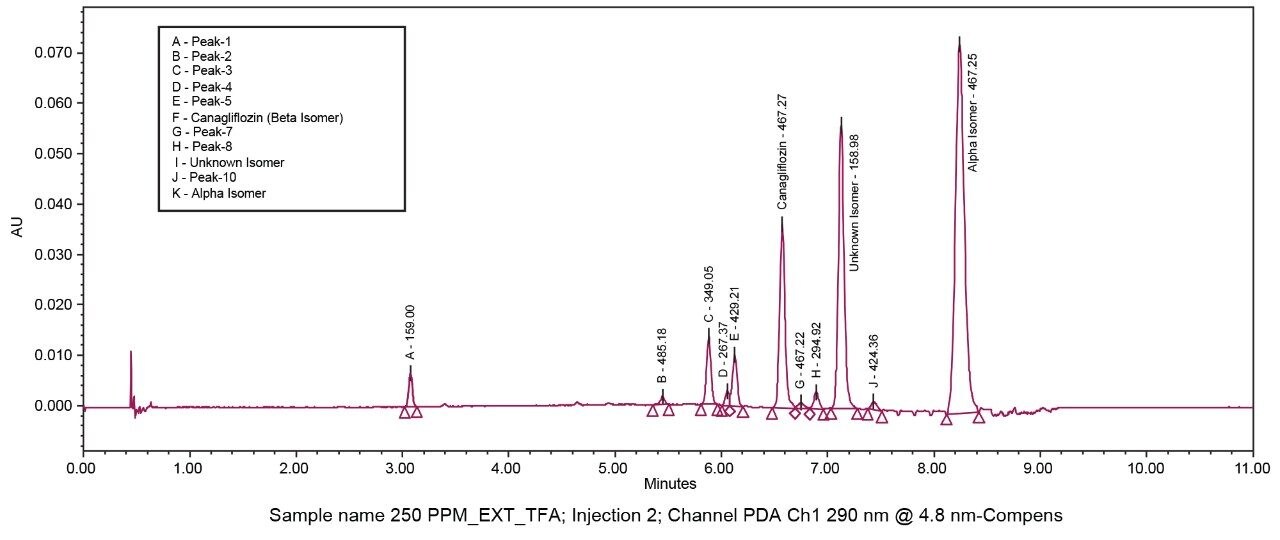
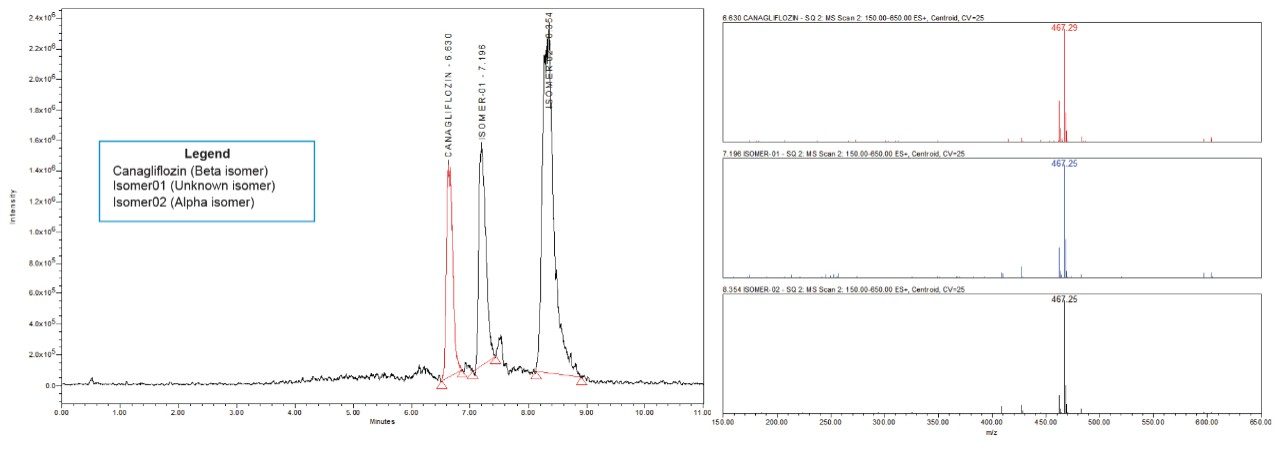
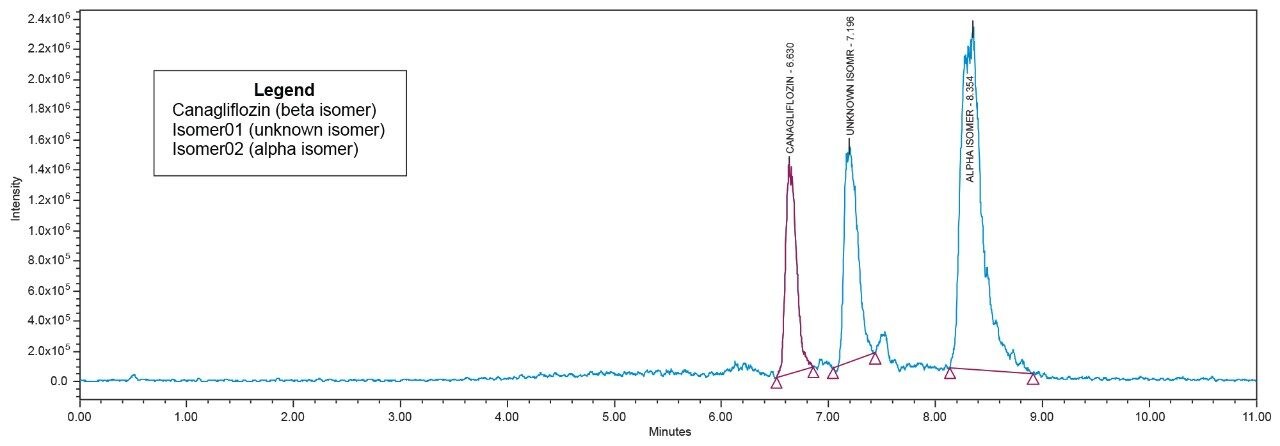
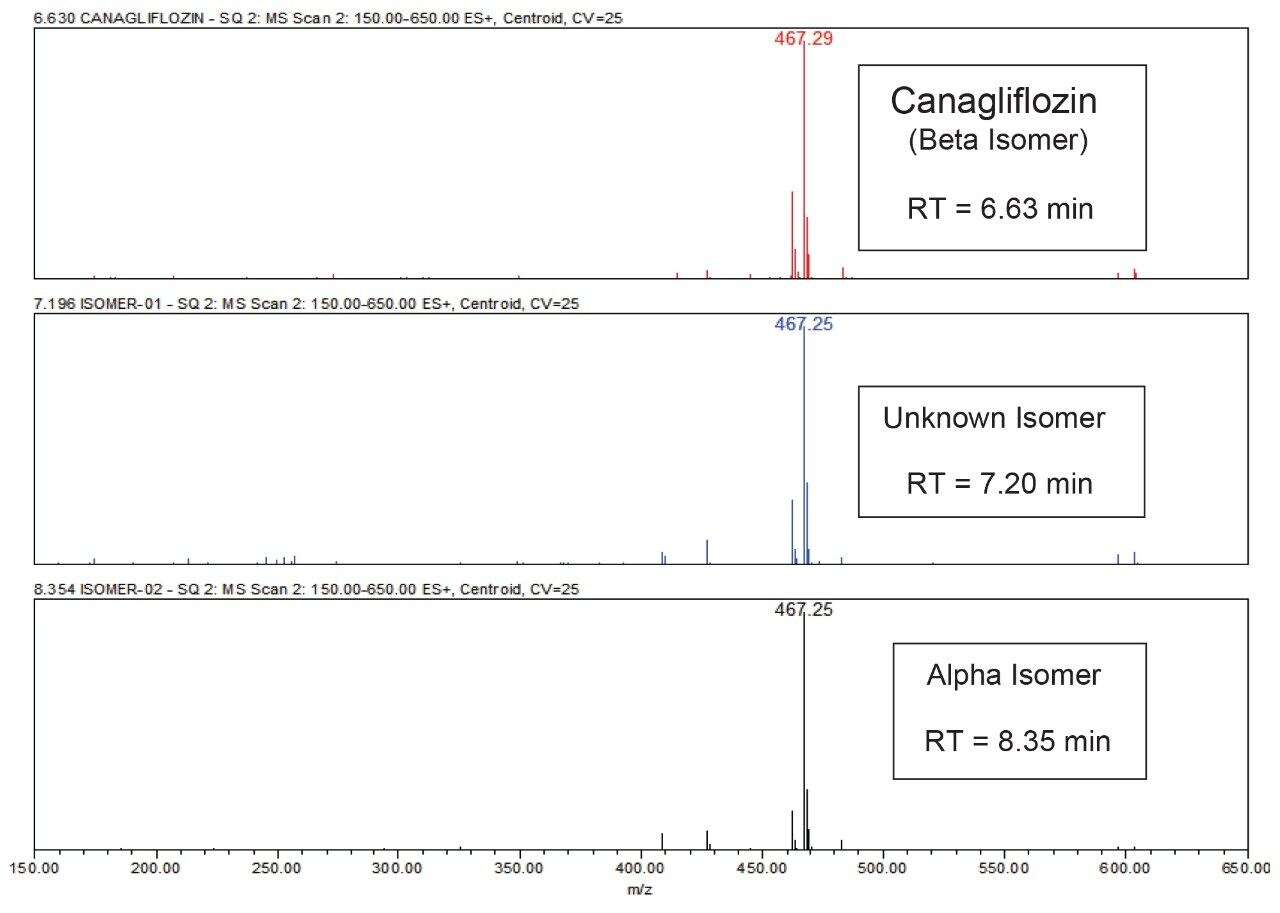
All the isomeric peaks were identified by orthogonal mass detection technique (the retention time of Canagliflozin beta isomer, unknown isomeric impurity, and alpha isomer were 6.6 min, 7.2 min, and 8.4 min respectively) as the sodium adduct in ESI positive mode (467 m/z for 444 Da of Molecular weight) [Figure 7].
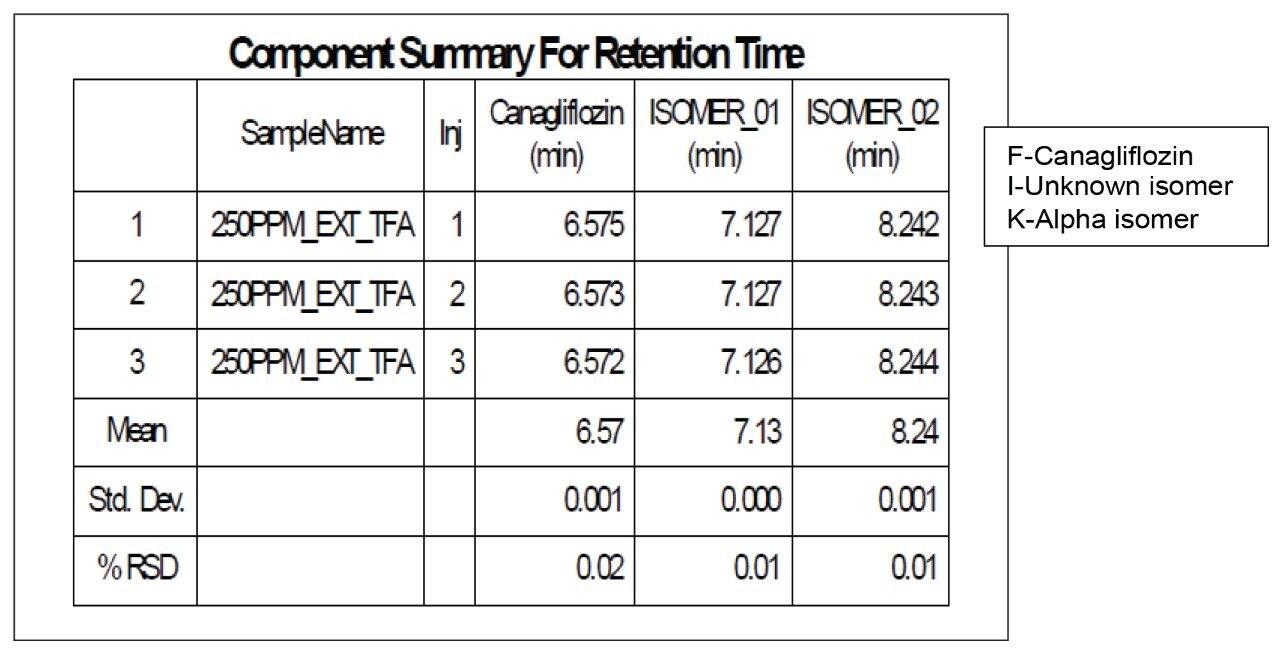
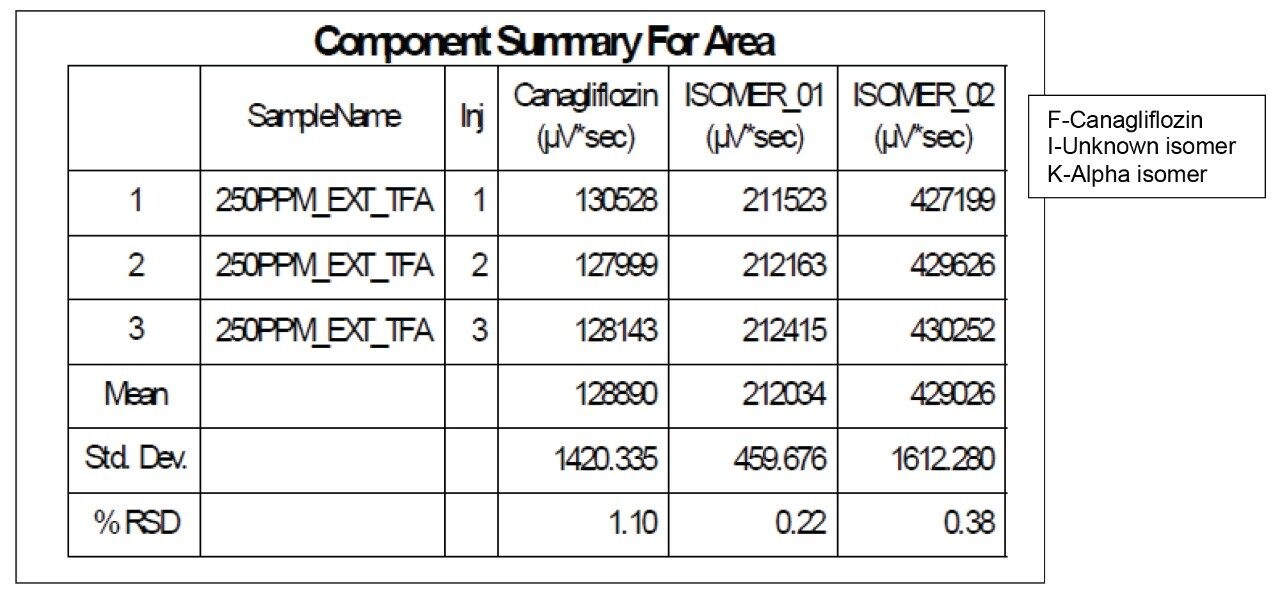
Chromatography shows excellent reproducibility with three replicate injections in terms of %RSD of area and retention time (Figure 8 and Table 2 & 3).
ACQUITY UPC2 technology enabled rapid and faster separation of isomeric impurities of Canagliflozin with a short run time of 11 minutes when compared to longer reverse phase chromatography of 65 minutes. The method provides excellent selectivity for the targeted impurity peaks, separation, and fast turnaround time with much lesser consumption of solvent system when compared to conventional normal or reserve phase methods. The ACQUITY UPC2 method can potentially be used for the chiral analysis of Canagliflozin and its isomeric impurities during product development stage and can be used as a tool for faster screening of method that will serve the purpose of easy scale up to Prep system for isolation and collection of complex isomeric entities.
720006138, November 2017