ACQUITY UPLC I-Class/Xevo TQD IVD System: Analytical Performance for Progestogens and Androgens
For in vitro diagnostic use. Not available in all countries.
Introduction
The Waters ACQUITY UPLC I-Class/Xevo TQD IVD System enables the quantification of organic compounds in human biological liquid matrices.
This document describes a test of the analytical performance of the ACQUITY UPLC I-Class/Xevo TQD IVD System for the analysis of testosterone, androstenedione, 17-hydroxyprogesterone (17-OHP), and dehydroepiandrosterone sulfate (DHEAS) in serum.

Experimental
The ACQUITY UPLC I-Class/Xevo TQD IVD System was controlled by MassLynx IVD Software (v4.1) and the data processed using the TargetLynx Application Manager. Calibrators and Quality Controls were prepared by spiking commercially available reference material in stripped serum and the samples were processed using the following conditions:
Sample Preparation Conditions
100 μL sample was precipitated with methanol, diluted with water, and centrifuged. Samples were loaded onto Oasis PRiME HLB μElution plates, washed, and eluted prior to analysis.
LC Conditions
|
Column: |
ACQUITY UPLC HSS T3 (IVD) 1.8 μm, 2.1 mm × 50 mm |
|
Pre-column: |
VanGuard HSS T3 1.8 μm, 2.1 mm × 5 mm |
|
Mobile phase A: |
2 mM ammonium acetate + 0.1% formic acid in water |
|
Mobile phase B: |
2 mM ammonium acetate + 0.1% formic acid in methanol |
|
Flow rate: |
0.6 mL/min |
|
Gradient: |
45% B over one minute, 45–65% B over 2.5 minutes, 98% B for 0.5 minutes |
MS Conditions
|
Resolution: |
MS1 (0.75 FWHM), MS2 (0.75 FWHM) |
|
Acquisition mode: |
MRM |
|
Polarity: |
ESI(+/-) |
Results and Discussion
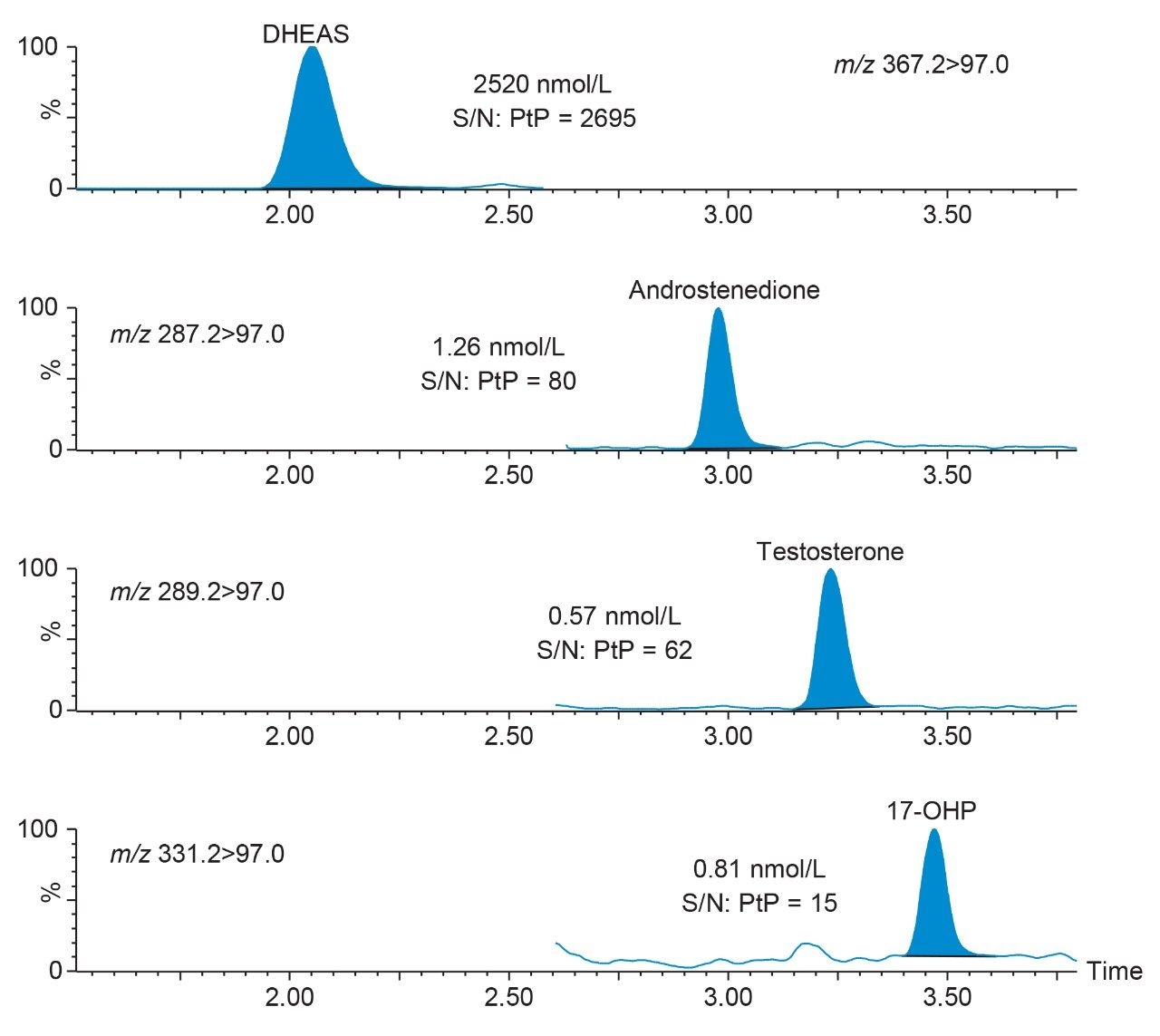
Performance characteristics of the steroid hormones on the Waters ACQUITY UPLC I-Class/Xevo TQD IVD System are shown in Table 1. Analytical sensitivity of the system for analyzing the steroid hormones in plasma is illustrated in Figure 1.
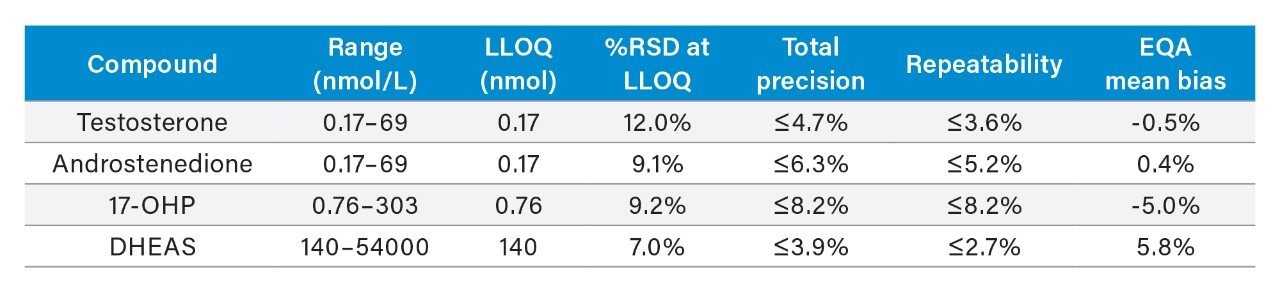
Note: To convert SI units to conventional mass units divide by 3.470 for testosterone (nmol/L to ng/mL), 3.494 for androstenedione (nmol/L to ng/mL), 3.028 for 17-OHP (nmol/L to ng/mL) and 2.716 for DHEAS (nmol/L to ng/mL).
Conclusion
The Waters ACQUITY UPLC I-Class/Xevo TQD IVD System has demonstrated the capability to deliver analytically sensitive, selective performance with excellent precision and accuracy for testosterone, androstenedione, 17-OHP, and DHEAS in serum.
Disclaimer
The analytical performance data presented here is for illustrative purposes only. Waters does not recommend or suggest analysis of the analytes described herein. These data are intended solely to demonstrate the performance capabilities of the system for analytes representative of those commonly analyzed using liquid chromatography and tandem mass spectrometry. Performance in an individual laboratory may differ due to a number of factors, including laboratory methods, materials used, intra-operator technique, and system conditions. This document does not constitute a warranty of merchantability or fitness for any particular purpose, express or implied, including for the testing of the analytes in this analysis.
720006355, August 2018