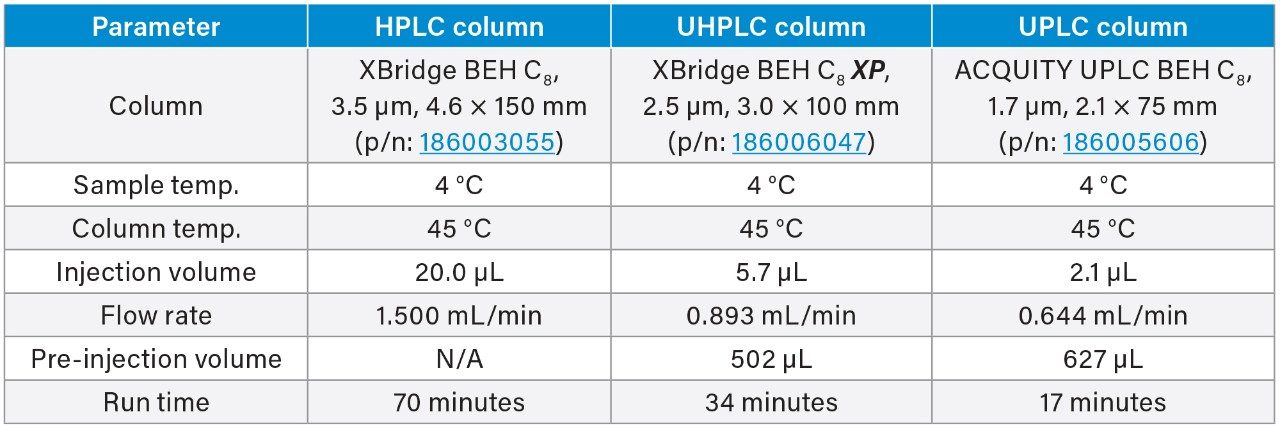
In this application note, the USP impurity monograph for quetiapine fumarate will be analyzed on the ACQUITY Arc System and then scaled to smaller particle sized columns using the Waters Columns Calculator.
Modernization of older high performance liquid chromatography (HPLC) methods, including scaling or transferring1 a method to new column or liquid chromatographic (LC) technologies can be considered a part of pharmaceutical lifecycle management.2 Many of these methods use >3 μm particle columns combined with high flow rates, which can result in long runtimes and high solvent consumption. Geometrically scaling HPLC methods to columns with smaller particle sizes can increase throughput while maintaining analytical performance. System dispersion and pressure limit are two factors to consider when scaling a method on a single instrument. Specifically, the LC system dispersion may impact the overall method performance and the LC system operating pressure may impose a physical limitation. Once the column dimensions and particle size are adjusted, the method requires the proper scaling of various method parameters including flow rate, injection volume, and gradient timing.
In this study, the USP impurity monograph for quetiapine fumarate3 will be analyzed on the ACQUITY Arc System and then scaled to smaller particle sized columns using the Waters Columns Calculator. Using the resolution, tailing factor, percent RSDs and the percent impurity calculation specified in the monograph,3 the scaled methods will be compared to the original HPLC method to ensure no loss of chromatographic or quantitative performance. The scaled methods provide decreased run times, lower solvent consumption, and overall increased sample throughput without changing the LC system.
The quetiapine fumarate standard (catalog #1592704), and the quetiapine system suitability standard (catalog #1592715) were purchased from the United States Pharmacopeia (USP). The unknown quetiapine fumarate sample was purchased from Alibaba.com.
All solutions were prepared according to the USP monograph.3 The system suitability and standard solutions were prepared in diluent comprised of Solution A and Solution B (86:14). The unknown sample solution was prepared in Solution A.
The concentrations of the solutions are 1.0 mg/mL for the system suitability solution, 0.001 mg/mL for the standard solution, and 1.0 mg/mL for the unknown sample solution.
|
System: |
ACQUITY Arc (Path 2) with active solvent preheating (CH-30A) and 2998 PDA Detector |
|
Mobile phase A: |
Solution A: Acetonitrile and buffer (25:75) |
|
Mobile phase B: |
Solution B: Acetonitrile |
|
Buffer: |
3.1 g/L of ammonium acetate in water. Two mL of 25% ammonium hydroxide was added to each 1 liter of solution. The final pH is not less than (NLT) 9.2 |
|
PDA wavelength: |
250 nm at 4.8 nm resolution |
Chromatography data software: Empower 3 FR 3
When scaling a method on a single instrument, the extra-column dispersion can impact the performance of the method. To maximize column efficiency, it should be paired with an LC system whose dispersion is small enough that it minimizes the extra-column dispersion or band broadening effects. However, optimal column performance, in terms of narrowest peak widths and highest peak capacity, may not be required for an analysis. It may be possible to use a small particle size column on a UHPLC, or even an HPLC system, and still meet method requirements. For example, the dispersion of the ACQUITY Arc System allows for suitable UHPLC analysis of a range of column particle sizes while maintaining the method requirements for the gradient quetiapine impurity method.
In addition to extra-column dispersion, it is important to consider the operating pressure limit of the LC system. When column particle size is decreased, the resulting backpressure generated is increased on the LC system. Therefore, geometrically scaling some methods may not be possible due to the pressure limits of the LC system. The Waters Columns Calculator4 can provide an estimated system pressure for a scaled method, but the actual pressure will be determined when running the scaled method on the LC system.
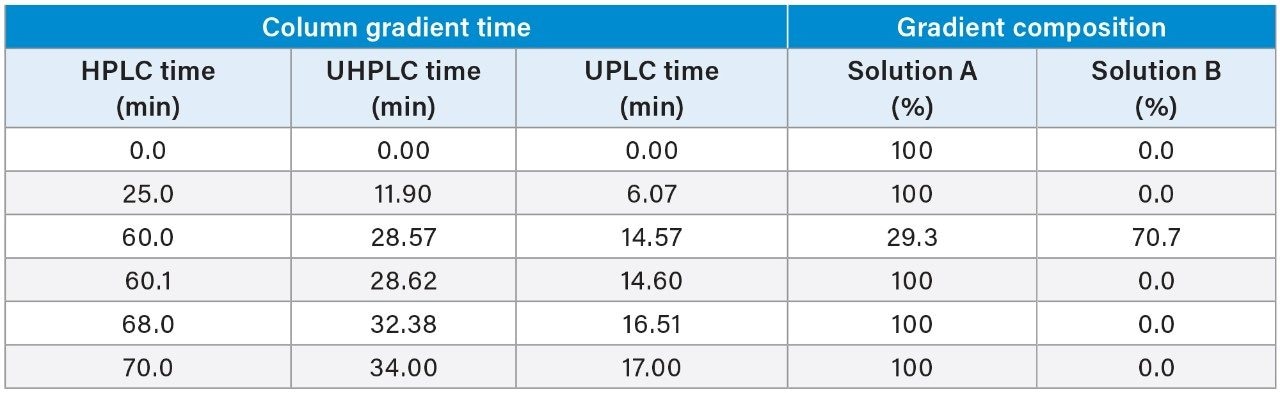
The quetiapine fumarate impurities USP method was first analyzed on the ACQUITY Arc System using the described monograph conditions.3 Performance was assessed based on the system suitability requirements as outlined in the monograph, which include resolution, tailing, and RSD for peak retention time and area. Then the column dimensions and method conditions were geometrically scaled to 2.5 and 1.7 μm particle columns.5
The scaled UHPLC and UPLC column dimensions were determined by maintaining the L/dp ratio of the original column, where L is the length of the column and dp is the diameter of the particle size. Therefore, a 2.5 μm, 3.0 × 100 mm, and a 1.7 μm, 2.1 × 75 mm column was chosen. To adjust the columns flow rate, injection volume, and gradient steps, the Waters Columns Calculator was used (Figure 1). When analyzed on the LC system, the scaled conditions for both methods did not exceed the pressure limits of the ACQUITY Arc System.
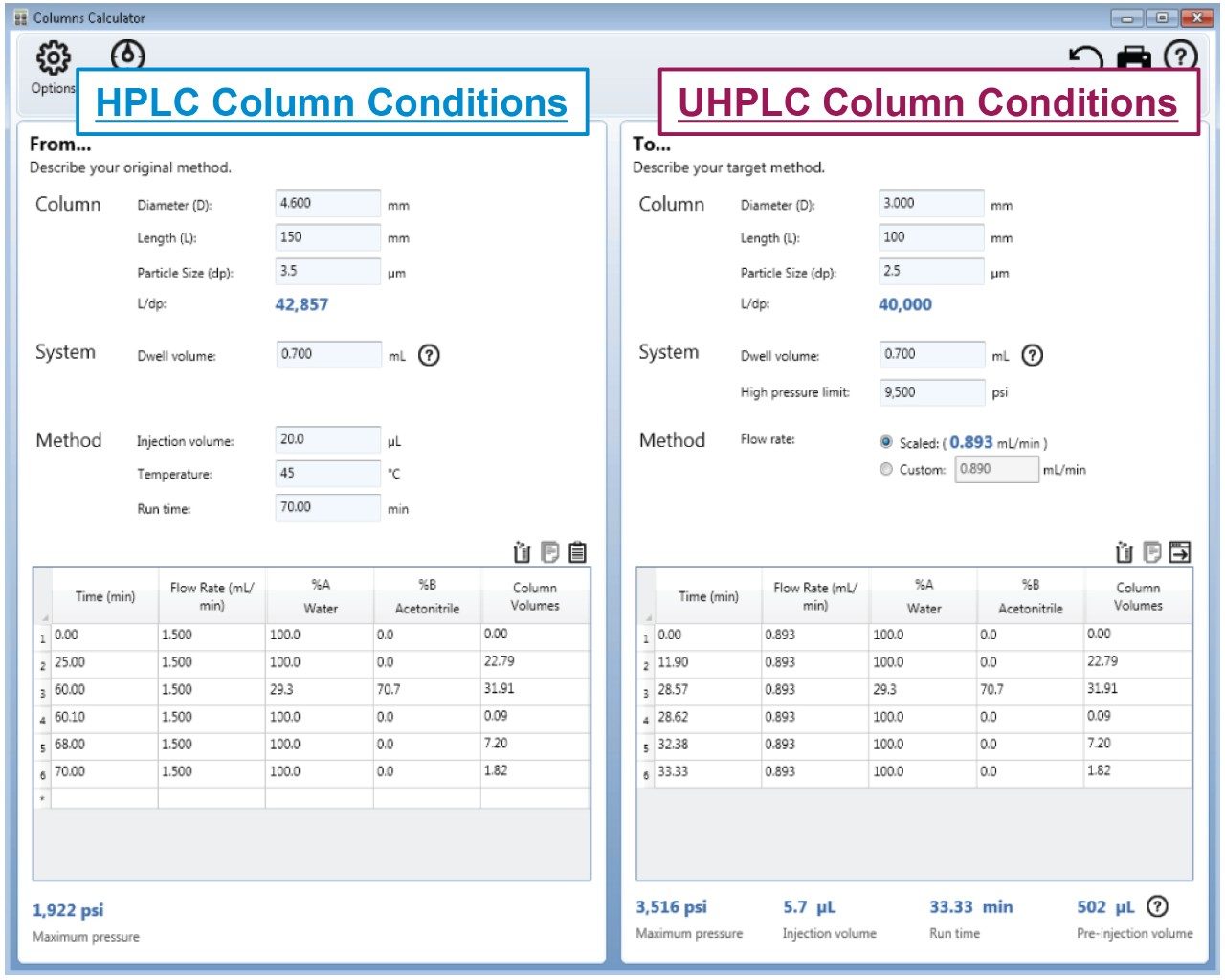
During a gradient method there is a delay between the time the mobile-phase composition is changed at the pump to when it reaches the head of the column. When scaling gradient methods, it is important to maintain this delay so that there is no change to selectivity of early eluting compounds. To account for this, the Waters Columns Calculator determines the “Pre-Inject” volume based upon the entered system dwell volume and the column dimensions. The “Pre-Inject” volume is calculated to be 502 μL for the 2.5 μm particle column method and 627 μL for the 1.7 μm particle column method6,7 (Figure 1).
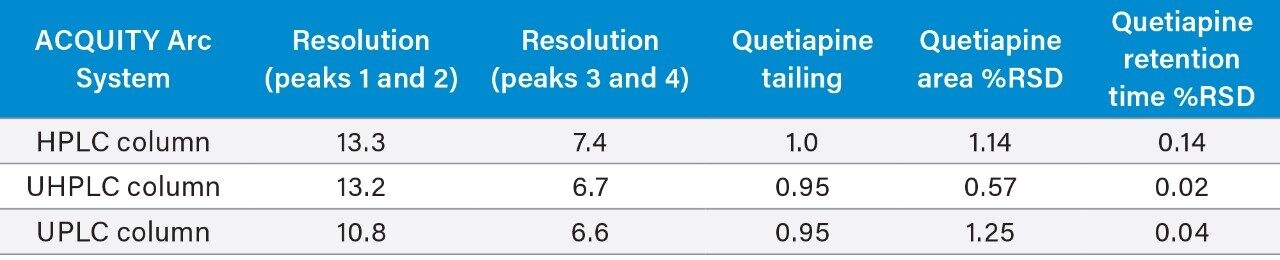
The two standard solutions and the unknown sample were prepared as described above with six replicate injections performed for each solution. The original HPLC method and the two scaled methods all show similar chromatographic performance (Table 1) in terms of resolution, tailing, and peak area and retention time RSDs. The resolution of peaks 1 and 2, was slightly lower for the UPLC method, likely due to the system dispersion which is not optimal for 1.7 μm particle columns. Although there is a small decrease, the resolution is still well above the method requirements of 1.5. Chromatograms of the system suitability solution are shown in Figure 2.
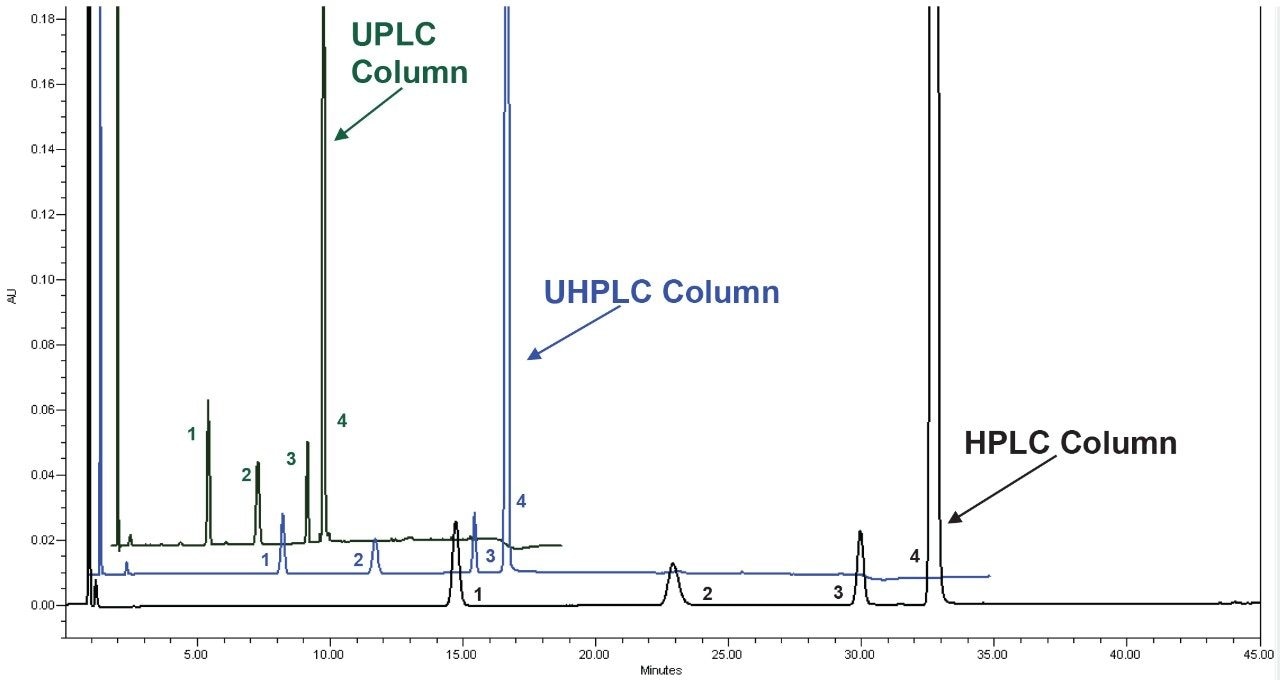
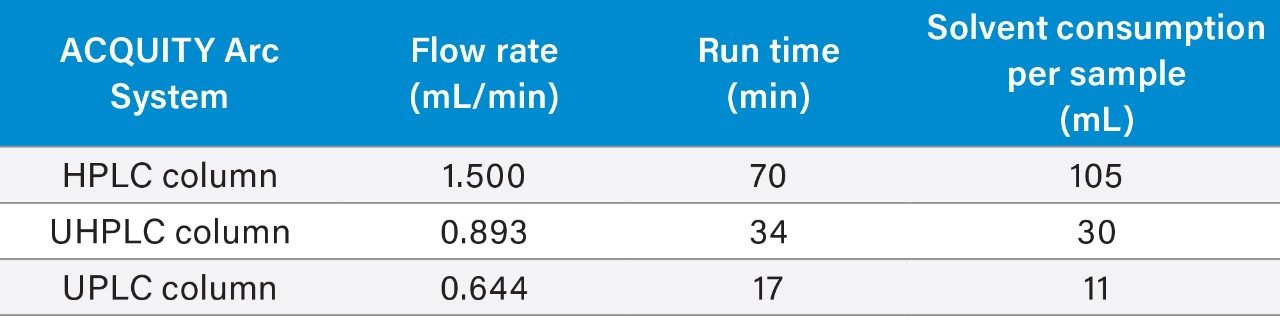
Scaling the original method to a smaller particle column significantly decreased the run time and solvent consumption (Table 2). For example, scaling the HPLC method to a 2.5 μm particle column decreased the run time by 51% and the solvent usage by 71%. Scaling the method to a 1.7 μm particle column decreased the run time by 75% and reduced the solvent usage by 89%.
To evaluate the quantitative reproducibility of the impurity method, the impurity content of the unknown sample was determined (Figure 3) using:
Result = (ru/rs) x (Cs/Cu) × (1/F) × 100
where ru is the peak response of each impurity from the sample solution, rs is the peak response of quetiapine from the standard solution, Cs is the concentration of USP quetiapine fumarate standard in the standard solution (mg/mL), Cu is the concentration of quetiapine fumarate in the sample solution (mg/mL), and F is the relative response factor for the impurity peak provided in the monograph.3
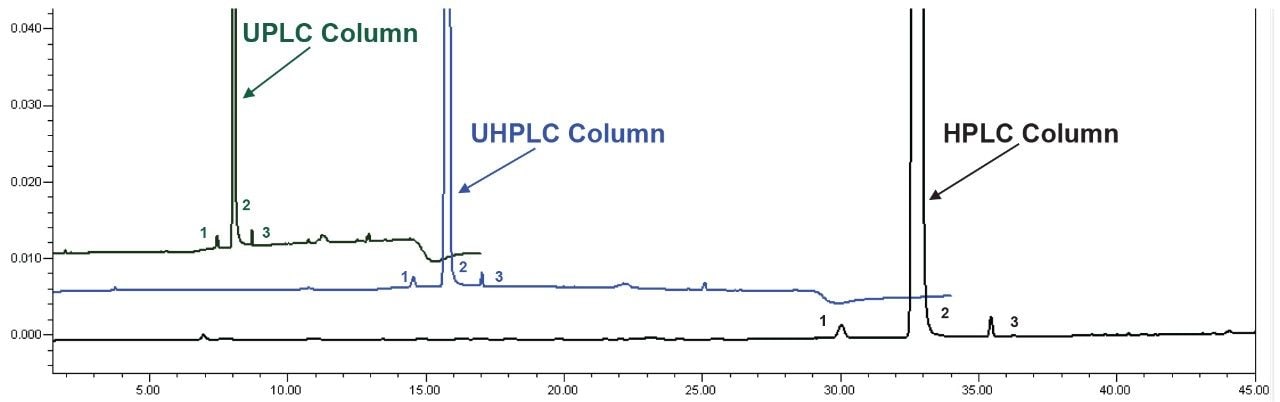
Two impurity peaks were found in the unknown sample, quetiapine desethoxy and an unknown impurity. The calculated percent of each impurity, as well as the total amount of impurities in the sample, can be found in Table 3. The quantitative results for the impurities contained in the active pharmaceutical ingredient (API) sample were consistent regardless of which method/column was employed on the ACQUITY Arc System.
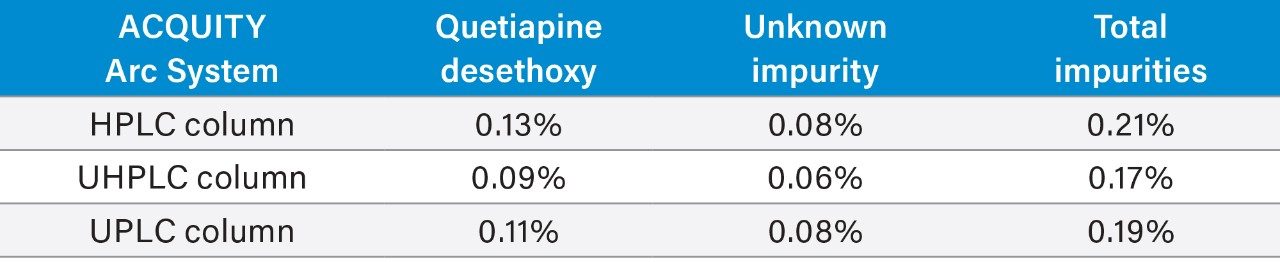
When a method is adjusted, it is important that the same analytical results are generated using the new method conditions. Scaling the USP quetiapine fumarate impurities method across the different column categories produced equivalent quantification of impurities contained within an unknown sample of the API.
When a laboratory has limited LC systems, it is possible to modernize methods by scaling traditional HPLC methods to columns with a smaller particle size and length. This was demonstrated on the ACQUITY Arc System using the USP quetiapine fumarate impurity gradient method. The original method was scaled from a 3.5 μm particle column to a 2.5 and 1.7 μm particle column using the Waters Columns Calculator. Without changing the ACQUITY Arc System, the HPLC, UHPLC, and UPLC column impurity methods provided similar chromatographic performance in terms of resolution, peak tailing, and retention time and peak area RSD. Additionally, quantitative results for impurities contained within an unknown API sample were consistent regardless of which method/column was used for analysis on the ACQUITY Arc System.
720006620, July 2019