In this application note, a common buffer system is used to screen mobile phase pH and ionic strength for size exclusion chromatography (SEC) and ion exchange chromatography (IEX) methods in a single chromatographic run.
Methods that demonstrate robustness and repeatability are critical in biopharmaceutical analyses. Although platform methods can offer a generalized “starting point” for analysis, screening multiple method parameters is often a necessary part of the method development process to gain a deeper understanding of target analytes and their physicochemical properties when present in increasingly complex samples. The Waters ACQUITY Arc Bio System is a modern LC platform that employs functionality to help scientists work more efficiently and effectively for developing higher quality products through enhanced instrumentation and methods.
This two-part application note series demonstrates how features of the ACQUITY Arc Bio System, such as column-switching capabilities and buffer preparation technology, can help streamline method development. In part 1, four RPLC columns were screened in series to evaluate differences in peak capacity and selectivity of targeted peptides across a diverse group of chemistries. In part 2, a common buffer system is used to screen mobile phase pH and ionic strength for size exclusion chromatography (SEC) and ion exchange chromatography (IEX) methods in a single chromatographic run. Auto•Blend Plus is a tool incorporated within Empower Software that uses four mobile phase stock solutions (acid, base, salt, and water) to program changes in mobile phase composition. This allows for adjustments to be made to pH and salt content without the need for manual buffer preparation. By using one set of buffers, a single experiment can be used to screen mobile phase conditions for both SEC and IEX by switching between two columns. This allows for a high-level screening protocol to be carried out in a timelier manner so that methods can be further optimized using a narrower and well-defined set of experimental conditions.
Formulated trastuzumab at 21 mg/mL was diluted to 10 mg/mL in water. Sample load was based on column care and use recommendations and was confirmed to be within the dynamic range of the optical detector (data not shown).
|
LC system: |
ACQUITY Arc Bio System with CM-A |
|
Detector: |
2489 UV/Visible (UV/Vis) Detector |
|
Columns: |
XBridge Protein BEH SEC, 200 Å, 2.5 μm, 7.8 mm × 150 mm (SEC); BioResolve SCX mAb, 3 μm, 4.6 mm × 50 mm (IEX) |
|
Wavelength: |
280 nm |
|
Injection volume: |
10 μL |
|
Column temp.: |
Ambient (SEC); 30 °C (IEX) |
|
Flow rate: |
0.500 mL/min (SEC) 0.750 mL/min (IEX) |
|
Mobile phase A: |
100 mM sodium phosphate monobasic |
|
Mobile phase B: |
100 mM sodium phosphate dibasic |
|
Mobile phase C: |
1 M sodium chloride |
|
Mobile phase D: |
Water |
|
SEC method: |
Auto•Blend Plus was used to deliver 20 mM phosphate; pH and salt concentrations are indicated in figures (15-min isocratic run) |
|
IEX method: |
Auto•Blend Plus was used to deliver 20 mM phosphate and a 25–125-mM linear salt gradient over 10 min; pH is indicated in figures (30-min run) |
*Please note that phosphate containing buffers should be evaluated with the intended analyte when using the BioResolve SCX mAb Column as performance may vary.
Empower 3 Chromatography Data Software SR2, FR4
An ACQUITY Arc Bio System was configured with a CM-A column compartment module, which houses the valves for column selection between the SEC and IEX columns or up to four 50-mm-long columns (Figure 1). The quaternary pump enables the use of Auto•Blend Plus, which is a buffer preparation technology capable of blending mobile phase compositions at various pH and salt concentrations using concentrated stock solutions.1 From Figure 2, flow is directed from the injection valve through one of the columns to the optical detector. Column selection is indicated by the user in the instrument method or selected in the Empower console so that valve switching is carried out automatically by the software.

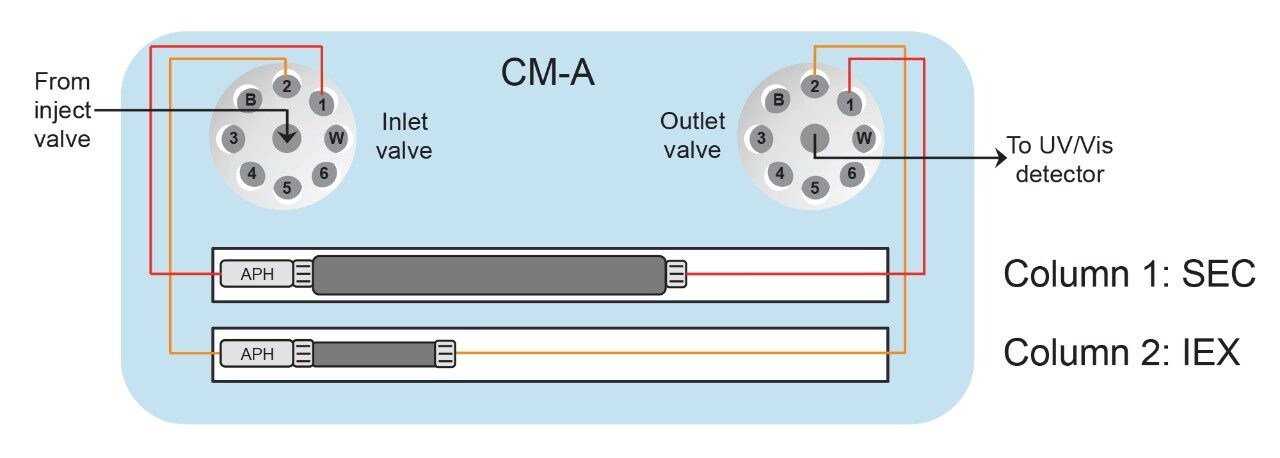
Both SEC and IEX parameters can be screened within a single sample set using a common buffer system, where Auto•Blend Plus eliminates the need to prepare each set of mobile phase conditions independently. To demonstrate this functionality, pH and ionic strength were screened using stock solutions to determine their impact on SEC of a monoclonal antibody (trastuzumab). After the final SEC run, the column was flushed with water for one hour (which is intended for short-term storage only) before switching to the second column position for screening IEX at various pH conditions. Because methods are intended for rapidly screening many method conditions for higher throughput analysis, short column lengths were used. Should greater resolution be required, longer columns can be used to further develop methods once method parameters have been narrowed down from the screening protocol.
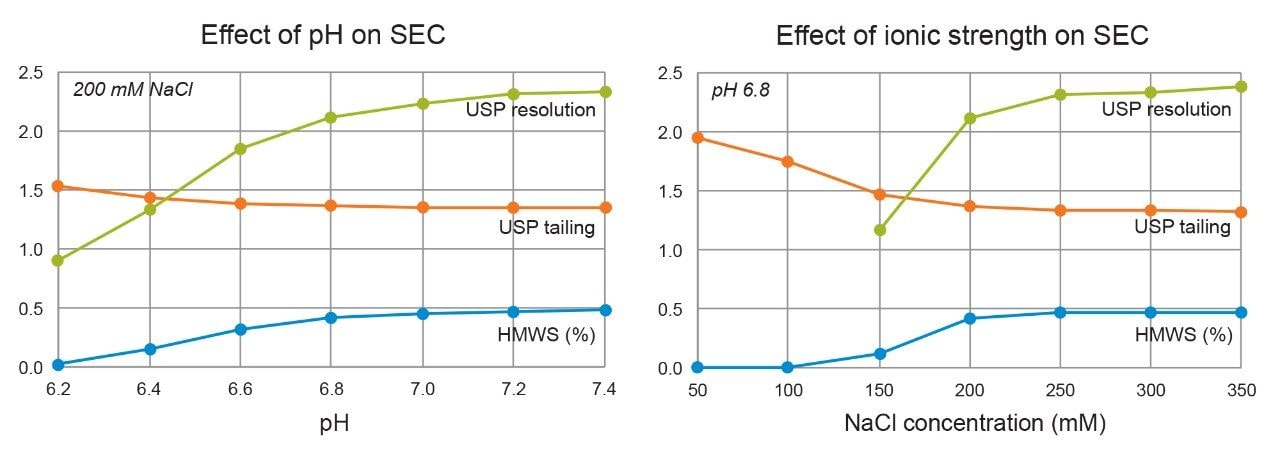
Mobile phase ionic strength and pH are important considerations when developing SEC methods. Both parameters should ensure that there are no secondary interactions between the stationary phase of the column and the analyte. In general, a higher salt concentration can help improve peak shape and reduce peak tailing, but because high salt concentrations can potentially corrode stainless steel LC systems or shorten column lifetime, excessive salt concentrations should be avoided where possible.
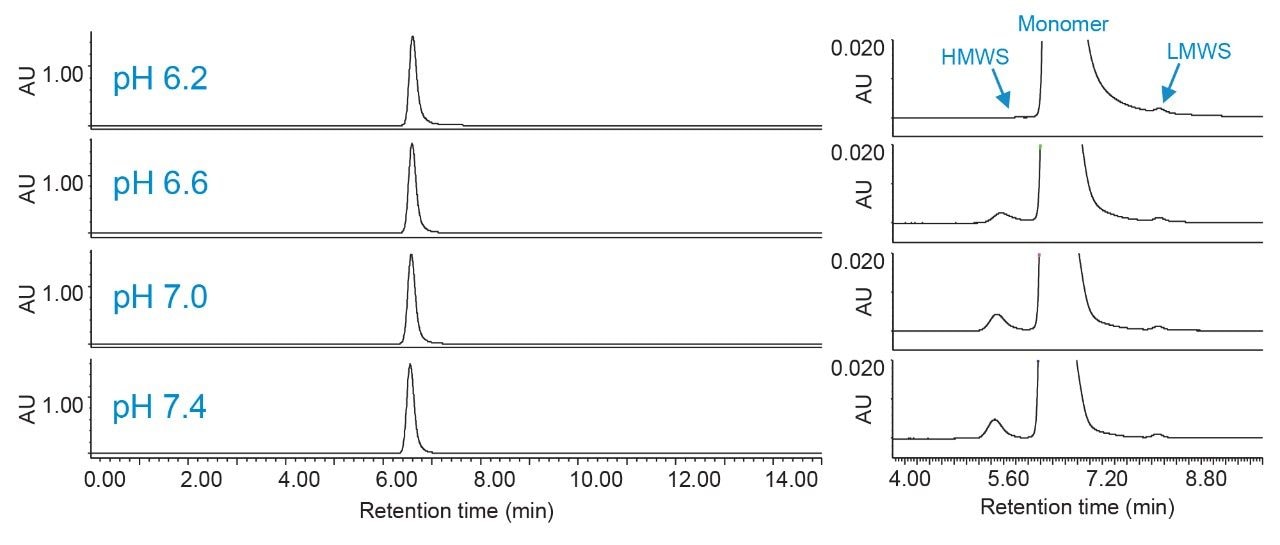
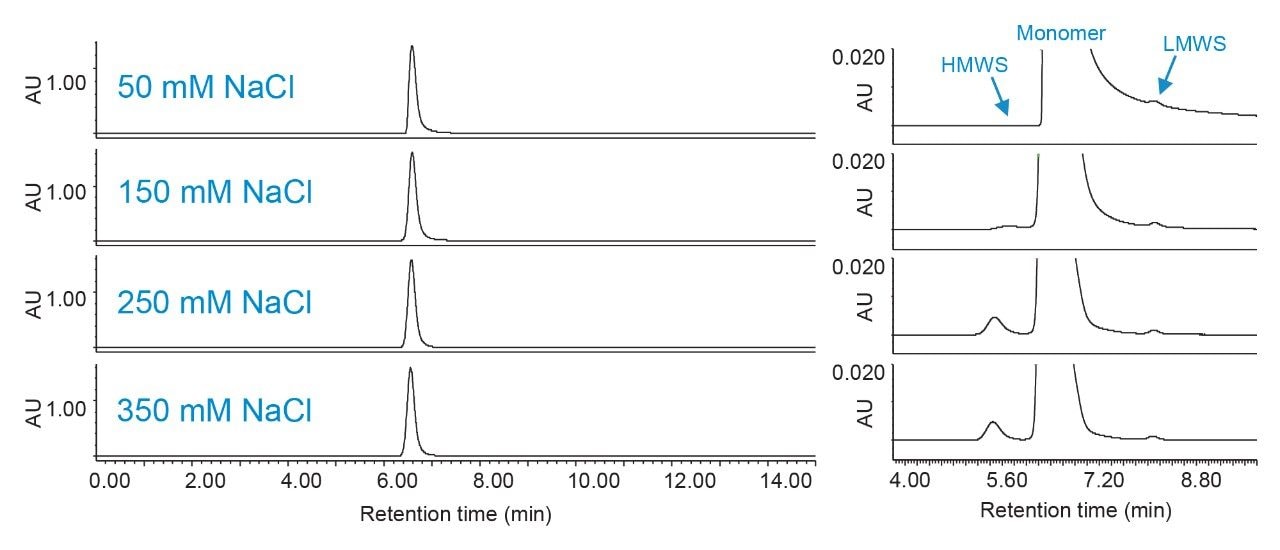
Figures 3 and 4 show the effects of pH and ionic strength on SEC of trastuzumab. Auto•Blend Plus was used to deliver 20 mM phosphate and variable pH and salt concentrations as indicated in the respective figures. From the insets, aggregate, or high molecular weight species (HMWS), recovery is poor at low pH and ionic strength. When pH and ionic strength are increased, the monomer peak becomes visibly narrower and peak tailing is reduced. A fragment, or low molecular weight species (LMWS), is also observed but not evaluated in this work due to being present at such a low relative percentage. It should be noted that if the intended application is meant to evaluate all major product impurities, including an additional LMWS that co-elutes with the monomer peak under the current conditions, a longer column length will be required to achieve the necessary resolution. Figure 5 shows the HMWS peak area percent, USP tailing, and USP resolution between the HMWS and monomer peak under all conditions tested. Data was collected from pH 6.2 to 7.4 (0.2 pH increments) and 50 to 350 mM NaCl (50-mM increments). (Figures 3 and 4 are meant to show trending behavior and do not show all data points for simplicity purposes.) When determining final method conditions, it is important to establish aggregate recovery while minimizing tailing and enhancing resolution. From Figure 5, changes in aggregate recovery, tailing, and resolution are less notable from approximately pH 7.0 to 7.4 and 250 to 350 mM NaCl. From these conditions, the user can further optimize the method. In addition to adjusting instrument method parameters, evaluating additional pore sizes and column lengths are also good practice, especially in cases where analyte properties are unknown.
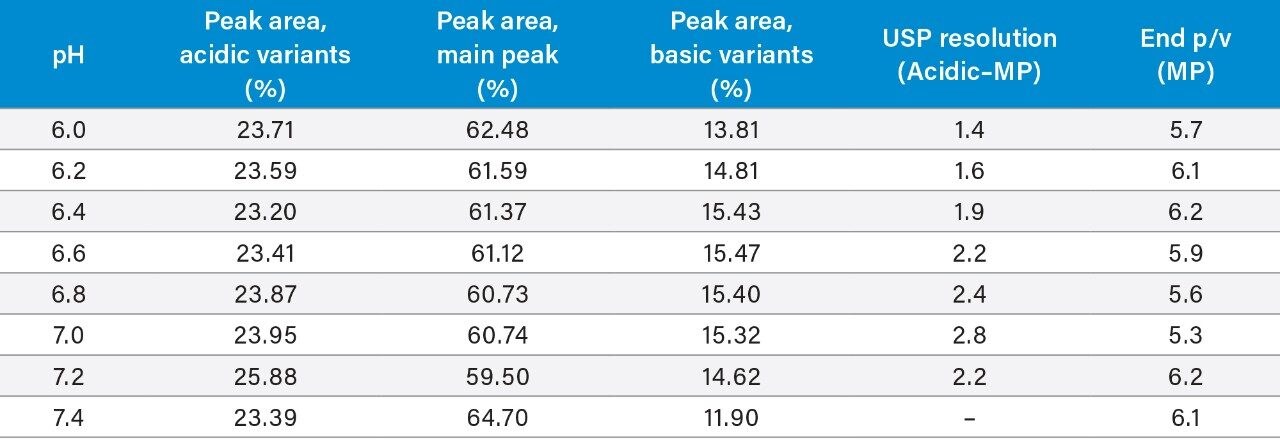
Although there are several parameters involved in optimizing IEX separations, it is important to understand the impact that pH has on selectivity. As previously mentioned, the column switching capability of the ACQUITY Arc Bio System allows users the flexibility to evaluate different techniques when using common mobile phase systems in conjunction with Auto•Blend Plus. Using the same mobile phase system, valve positions were switched to direct flow to the IEX column (Figure 2). Auto•Blend Plus was used to deliver 20 mM phosphate and a linear salt gradient from 25–125 mM NaCl over 10 minutes while holding pH constant throughout the run. Figure 6 shows the effect of pH on charge variant analysis of trastuzumab from pH 6.2 to 7.4 at 0.4 pH unit increments. (Data was collected for every 0.2 pH unit change and is further described in Table 1.)
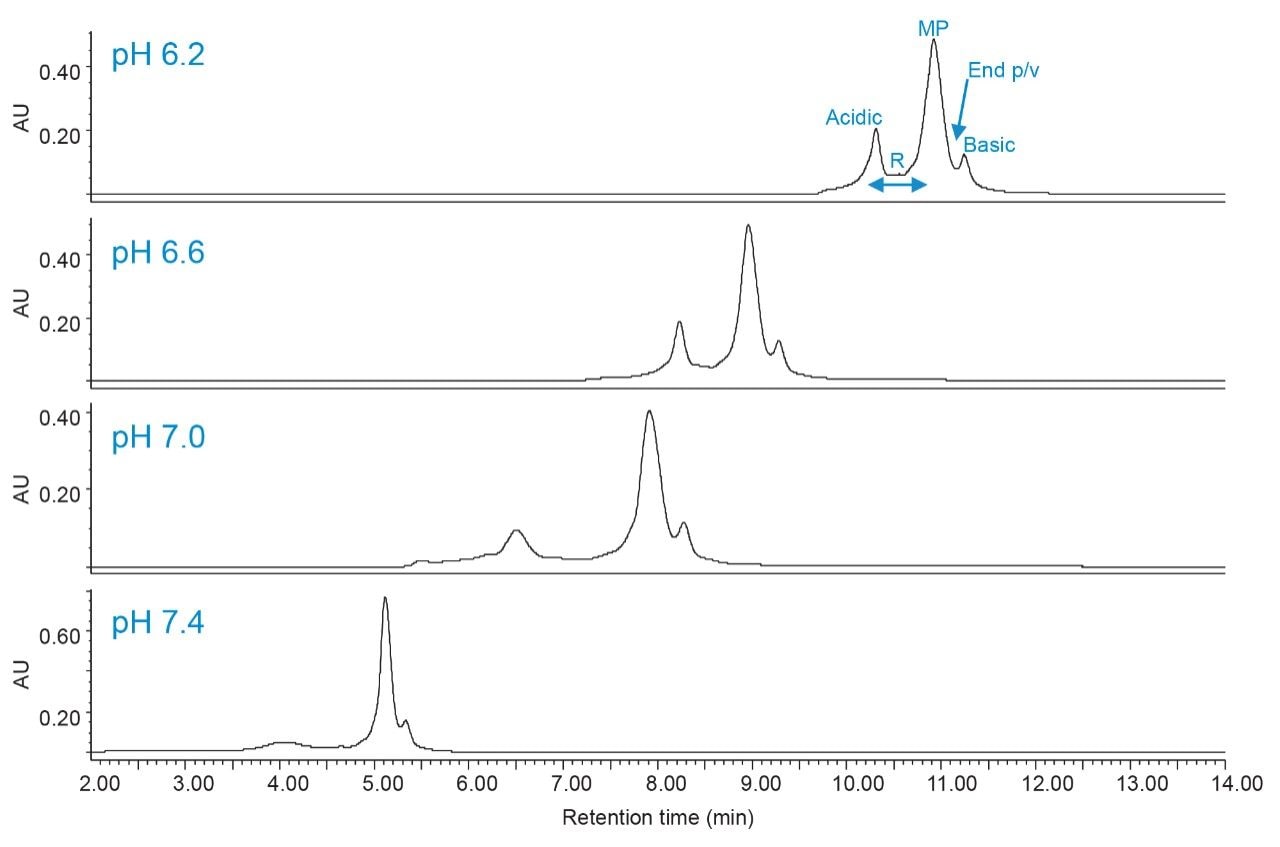
As expected, increasing the pH results in less retention, as the higher pH reduces the positive charge on the analyte and weakens the interaction with the stationary phase. From visual inspection of the data, similar chromatographic profiles are seen at lower pH. Ideally, an IEX method would be further developed in this lower pH region to ensure analyte stability. Because small changes in pH impact the chromatography at higher pH, the final method should account for small variations in pH due to different mobile phase preparations.
Further treatment of the IEX data evaluates peak area percentage of acidic and basic variants and the main peak, USP resolution, and peak height to valley height (p/v) (Table 1). This data, in combination with chromatographic results, suggest that further method development should be interrogated in the range of ~pH 6.2 to 6.6, where the method appears to be more robust. In addition to using a longer column, the salt concentration can also be narrowed to improve resolution, if desired, once an appropriate pH is determined. Salt concentration was not evaluated with the initial conditions screened as a user would have to evaluate the pH data to determine how to manipulate the salt gradient for best results.
In this work, column switching capabilities and buffer preparation technology were used to develop high-level screening protocols. Auto•Blend Plus was used to blend various mobile phase compositions from concentrated stock solutions so that method conditions could be readily screened without the need for preparation of separate mobile phases at each set of conditions. This allowed for a single sample set to be generated to screen pH and ionic strength for both SEC and IEX methods with minimal user intervention. The bio-inert flow path of the ACQUITY Arc Bio System is especially advantageous for applications such as these, which otherwise leave an LC system susceptible to corrosion due to the buffer systems used for analysis. These features of the ACQUITY Arc Bio System allow for a simplified and higher throughput approach to early method development, which can help guide further method optimization.
720006854, April 2020