
This application note demonstrates RP-HPLC method for purification of dually-labeled synthetic oligonucleotides.
Dually-labeled oligonucleotides are used mainly in quantitative polymerase chain reactions (qPCR), genotyping, and for diagnostic applications. Most common dually-labeled oligonucleotides are TaqMan probes, labeled with 5’FAM and 3’ TAMRA dyes. Synthesis of these oligonucleotides is challenging, especially when a “one-pot” approach is adopted without semi-product purification. A target product is contaminated with the partially labeled, non-labeled oligonucleotides, and excess of dyes. The impurities interfere with molecular biology assays and have to be re-moved.
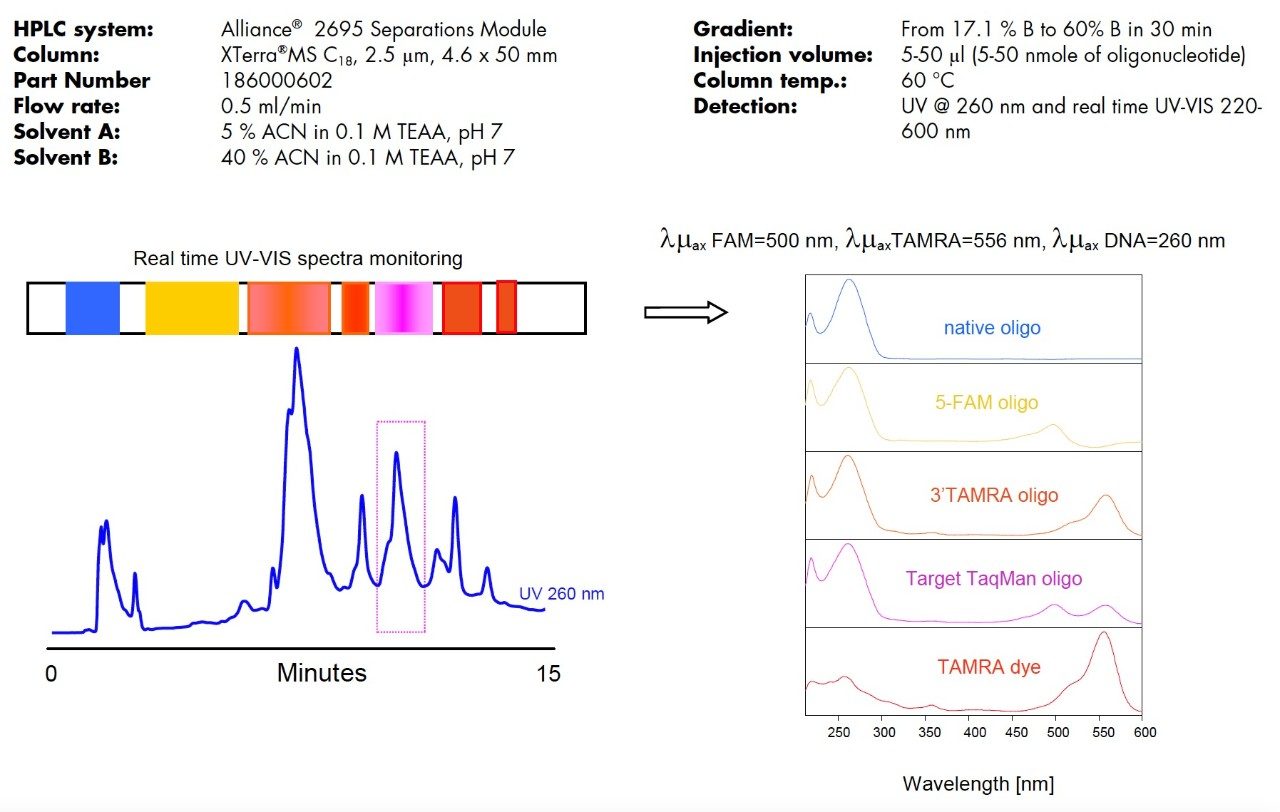
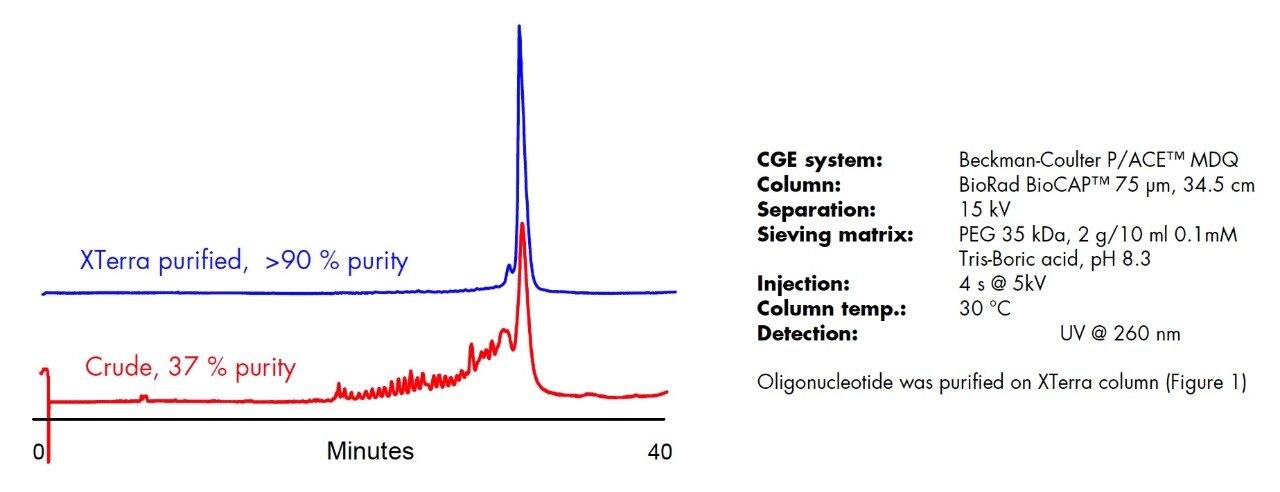
XTerra MS C18 Columns are packed with porous 2.5 μm, hybrid particles. The sorbent has extended stability at temperatures and pH’s typically used for oligonucleotide separations (50-60 °C; pH 7-9). Non-labeled oligonucleotides, singly-labeled failure sequences, and excess of free dyes can be easily removed from the target product using XTerra MS C18 Columns. Figure 1 shows the purification of 100 nmole of 31mer TaqMan oligonucleotide produced in a “one-pot” synthesis. Undesirable failed products can be can be successfuly removed from product using an XTerra Column. The mobile phases are volatile and can be removed from collected fractions by evaporation. The 4.6 x 50 mm column is suitable for purification of 50–200 nmole scale synthesis in a single injection. Larger amounts (200 nmole–1 μmole) of oligonucleotides can be purified using 10 x 50 mm column.
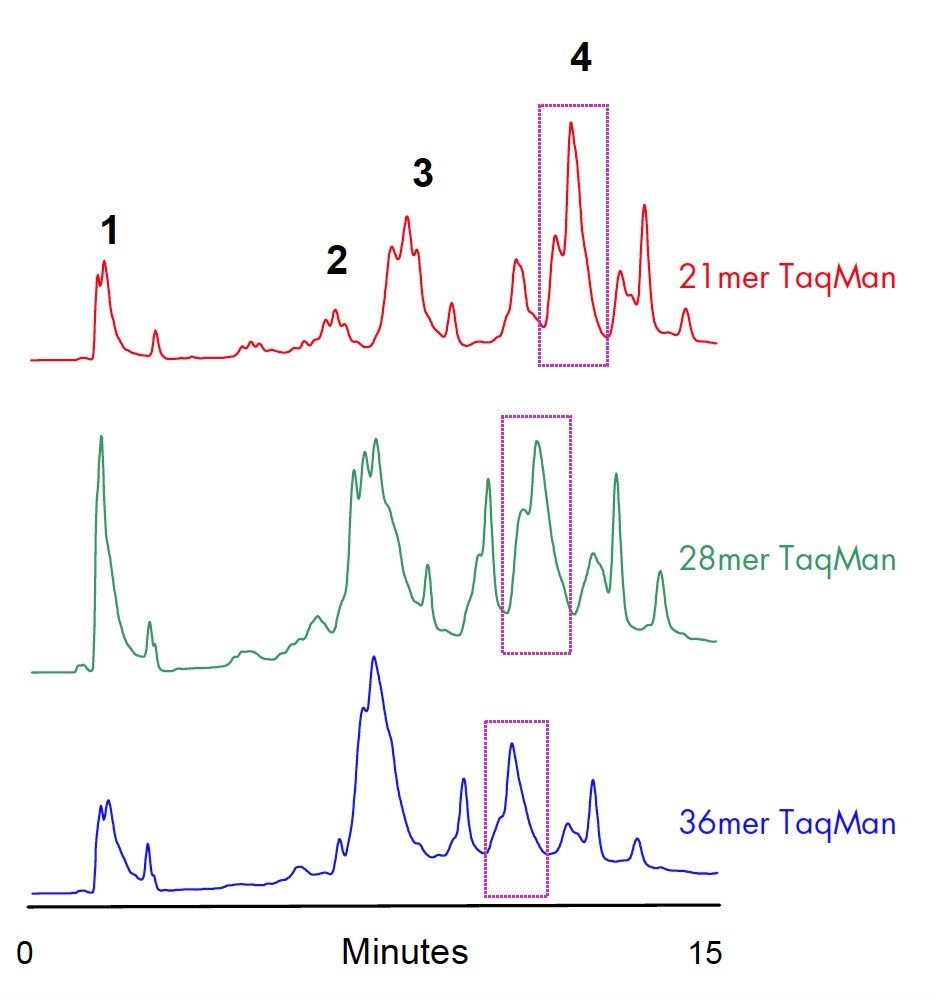
Retention of dye-labeled oligonucleotides is governed by the hydrophobicity the dye. This is demonstrated on the purification of three different TaqMan probes (Figure 3). Non-labeled oligonucleotides elute first (1), followed by FAM labeled failed sequences (2). TAMRA-only labeled failure fragments (3) elute before the dually-labeled target product (4). Other peaks are mostly un-conjugated TAMRA dye. Target product fraction appears to be a group of co-eluting peaks. The partial separation is caused by the differences in HPLC retention of dye isomers. Mass spectrometry analysis confirmed that the collected product is homogeneous (one molecular mass).
In order to select the collection time window, we utilized real-time UV-VIS spectra monitoring along with the 260 nm UV detection (Figure 1). The typical maxima at UV-VIS spectra indicated the elution of non-labeled, singly labeled and dually-labeled products (three λmax were present in the spectrum). Because of the similar pattern of elution, the purification of different length TaqMan probes becomes a routine for experienced user. Triethylammonium acetate/ACN buffer is volatile. Collected fractions are simply dried down and oligonucleotides are ready for assays.
WA20772, June 2003