This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the use of Waters SQ Detector 2 for LC-MS glycoprofiling of an intact 145 kD biotherapeutic monoclonal antibody.
Nominal mass quadrupole-based systems can be used for routine monitoring of biotheraputics, including protein mass confirmation and mAb glycovariant profiling.
The ability to derive semi-quantitative glycosylation profiles at the intact protein level permits rapid screening and monitoring of antibodies, with minimal sample preparation, and reduces the need for more granular but time-consuming approaches such as reduced antibody mass profiling or released glycan analysis.
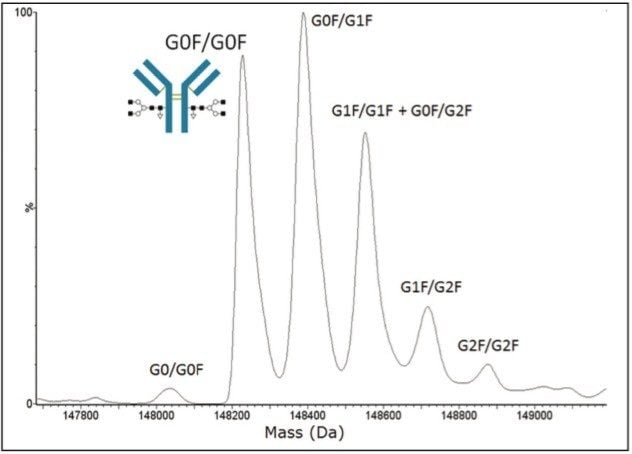
Glycosylation is known to play a critical role in stability and function of therapeutic antibodies. The abundance of the singly defucosylated glycovariant (Figure 2, labeled G0/G0F) has dramatic impact on resulting antibody-dependent cell-mediated cytotoxicity (ADCC) immune response, and is routinely screened for (or against) depending on the desired mode of action for the therapeutic. Other examples now populate the literature where the presence of extended sialic acid bearing glycovariants or relative proportionality of the neutral glycovariant structures can have demonstrable effects on antibody therapeutic stability, safety, and efficacy.
While exact mass Tof-MS has come to dominate the discovery workflows used for biopharmaceutical protein characterization, nominal mass quadrupole mass detectors have remained a workhorse for routine protein intact mass analysis. Here we explore the capabilities of the Waters SQ Detector 2 quadrupole mass analyzer for confirming the mass of an intact biotherapeutic antibody, and displaying the glycovariant profile for that protein.
LC-MS analysis of a therapeutic monoclonal antibody standard (50 μg, Waters Part No. 186006552) was acquired using a Waters ACQUITY UPLC H-Class System coupled to a SQ Detector 2. The antibody was loaded onto a 2.1 x 50 mm Protein Separations Technology (BEH 300Å C4, 1.7 μm) column (80 °C), desalted with a 0.5 mL/min flow of 5% acetonitrile in 0.1% formic acid for 1.5 min to waste, and then eluted with a 1.5 min linear gradient (5-95% acetonitrile, 0.1% formic acid, 0.2 mL/min) directed to the mass detector. Spectra were acquired (1 Hz, 500-3000 m/z) in the ESI+ mode of acquisition.
Spectra summed over the chromatographic peak of the desalted antibody (Figure 1) gave rise to the MaxEnt1 deconvoluted results shown in Figure 2. The major peaks in the deconvoluted mass spectrum corresponded to the antibody containing pairs (one per heavy chain) of the fucosylated biantennary glycan structures typically associated with monoclonal antibody N-glycoproteins, and an N-terminal pyroglutamic acid modification on each of the heavy chain subunits. The observed mass of the first major glycoform (G0F/G0F, containing two fucosylated N-glycan core structures without galactose extensions) was 148,227 Da, in good agreement (< 50 ppm error) with the calculated mass of the modified antibody (148,220 Da).
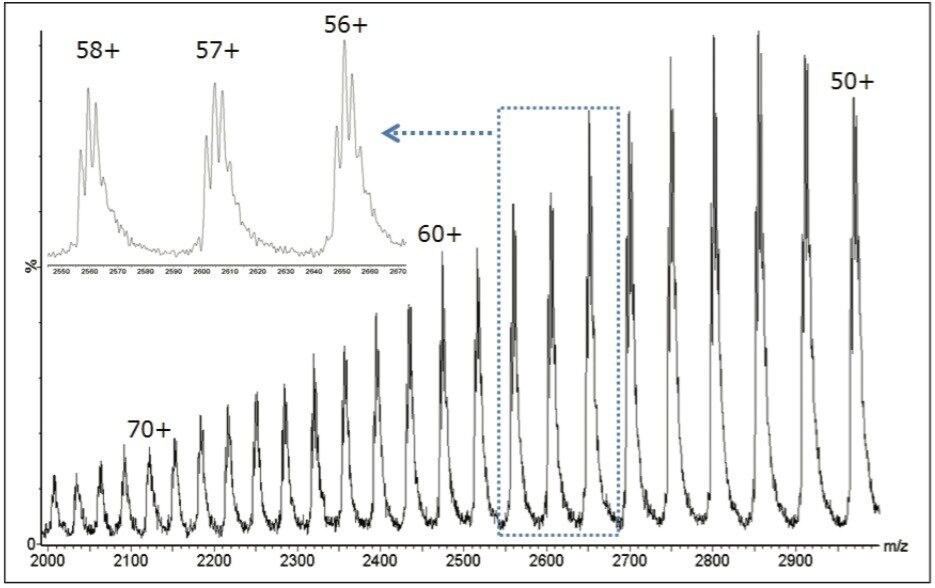
Notable in the raw (m/z) data, is that the mass spectral charge state envelope (roughly representing charge states 50+ through 70+) makes full use of the extended m/z range (up to 3000 m/z) of the SQD 2 platform. This is typical of larger proteins, and illustrates that measurement of ions at high mass-to-charge ratio is a fundamental requirement for quadrupole-based systems intended for intact biomolecule analysis.
As shown in Figure 1 (inset), tuning the SQ Detector 2 for unit resolution is sufficient to distinguish glycovariant structures within individual charge states. The exceptional performance of the MaxEnt1 deconvolution algorithm to infer a more highly resolved deconvolution spectrum from this multitude of charge states, represented over the larger spectral window, enables both qualitative glycan assignments and relative glycoform distribution determinations.
Work at the intact level can indicate the requirement for additional monoclonal antibody profiling. While generally applicable to most therapeutic antibodies, some with more extensive microheterogeneity (e.g., high levels of unprocessed C-terminal Lysines, inefficient N-terminal pyroglutamic acid formation, extensive oxidation) generally prove more amenable to profiling using chromatographically resolved light and heavy chains within a reduced antibody LC-MS analysis. Such information can often be inferred from intact antibody screening, and confirmed using the more granular reduced subunit analysis.
720004424, July 2012