Developing a Separation for Eleven Boronic Acids Using Maxpeak™ Premier Column Technology on an Arc™ HPLC System
Abstract
High Performance Liquid Chromatography (HPLC) has been a staple in analytical laboratories for several years. The utility of this technique is such that it can be used to analyze a wide variety of analytes. HPLC allows a lower cost alternative compared to UHPLC instruments, which boast higher performance for the higher capital investment. HPLC systems are still used regularly in the pharmaceutical industry for QA and QC type work such as batch release testing and method development.
This application note focuses on using an HPLC instrument to develop a separation of eleven boronic acid compounds. Boronic acids are used in synthetic chemistry to facilitate a variety of chemical reactions. They are also used as tags for compounds which are not readily detected via UV or fluorescence detection, such as sugar compounds. Final method conditions were realized using an XSelect™ Premier HSS T3 Column where full resolution was obtained for all eleven compounds.
Benefits
- Baseline resolution for eleven structurally similar boronic acids in a single run
- Column screening of five different HPLC columns to highlight selectivity differences
- Systematic screening protocol applied to streamline method development
Introduction
Boronic acids are a class of compounds used regularly in synthetic chemistry, as they participate in the Suzuki-Miyaura coupling reaction, which cross-couples a boronic acid to an organohalide. This allows creation of a variety of different bioactive molecules and enables the creation of compound libraries to study structure-activity relationships.1 Boronic acids can also be used to tag certain diol containing compounds.2 Their prevalence in these workflows necessitates analysis, whether that be as a part of labeling diol compounds, or as reaction monitoring for a coupling reaction. Having a method to separate the most common boronic acids is important as it can be used to monitor their use along with other reaction precursors, or final products.
To develop a method, eleven commonly used boronic acids were selected from literature searches and analyzed. Figure 1 shows the chemical structures of the eleven boronic acids tested in this application note. The method was developed using the previously documented systematic screening protocol, adapted for an Arc HPLC System with a single column heater and a PDA detector.3-5 The approach applies a tiered screen model which streamlines method development by eliminating the need for full factorial analysis while also breaking up the process into smaller and easier steps. This alleviates the stress of deciding the “best” conditions, as each step has clearly defined parameters. This method development approach has been successfully implemented several times and is further improved using MaxPeak Premier columns. MaxPeak Premier columns employ MaxPeak High Performance Surfaces (HPS) Technology, which eliminates non-specific adsorption of analytes onto the metal surfaces of the column such as the frits. This technology has shown to be extremely beneficial for acidic compounds containing phosphoric acid and carboxylic acid moieties as well as other metal sensitive compounds.6-8 Because of the success of this technology for other acid groups, MaxPeak Premier columns were used here to eliminate any non-specific adsorption (NSA) that may occur with these compounds. By eliminating NSA, we can ensure that any effects we see on the chromatography are related to the mobile phase and stationary phase alone, and not driven by surface-analyte interactions.
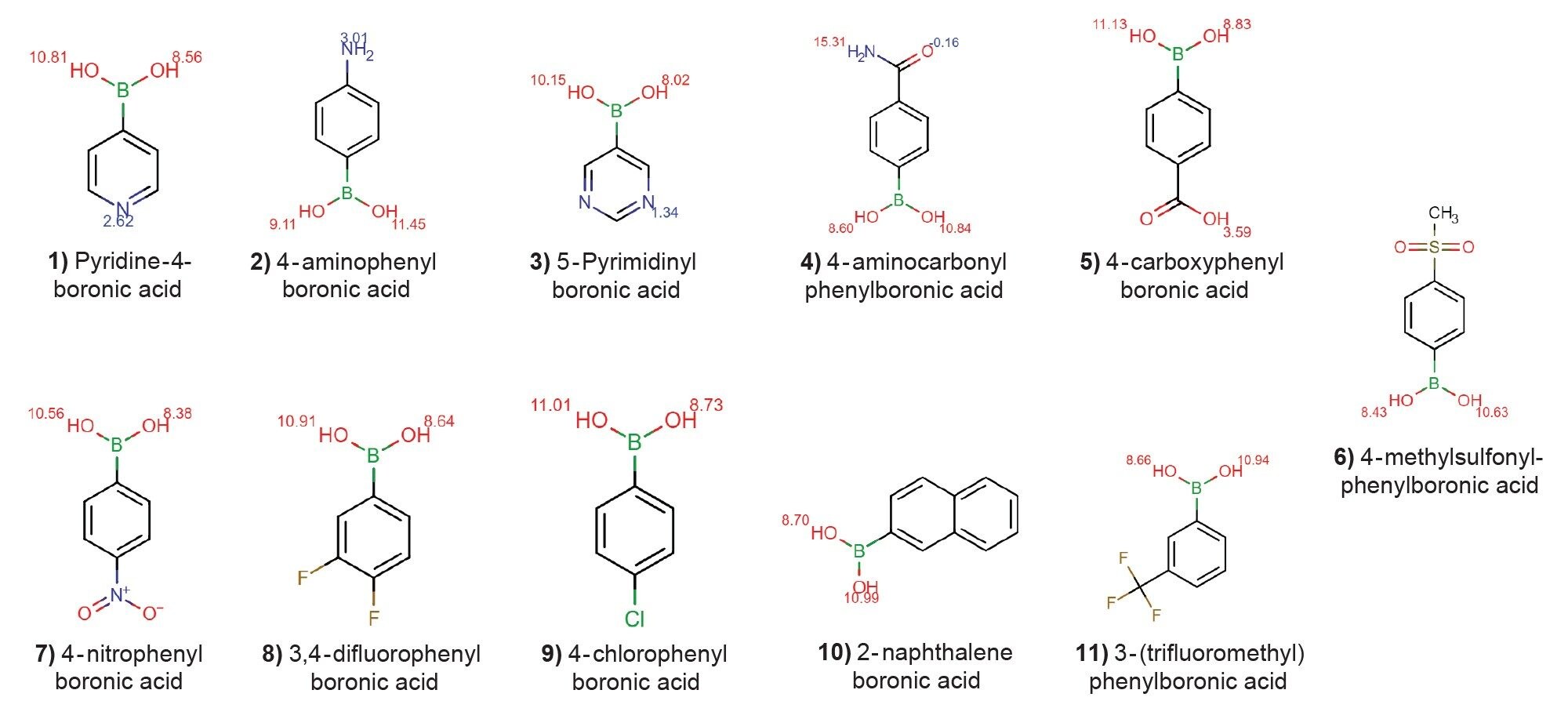
Method development was carried out over several days and a final method was determined and will be shown in this application note.
Experimental
Sample Description
Stock solutions of each boronic acid created at 1 mg/mL. Stock solutions combined to create a mixture at 0.09 mg/mL each boronic acid for analysis.
LC Conditions
|
LC system: |
Arc HPLC System with 2998 PDA Detector |
|
Detection: |
UV @ 254 nm |
|
Columns: |
XBridge™ Premier BEH™ C18, 4.6 x 100 mm, 3.5 µm (p/n: 186010660) XBridge Premier BEH Phenyl, 4.6 x 100 mm, 3.5 µm (p/n: 186010676) XBridge Premier BEH C8, 4.6 x 100 mm 3.5 µm (p/n: 186010951) XSelect Premier HSS T3, 4.6 x 100 mm, 3.5 µm (p/n: 186010935) XSelect Premier CSH™ C18, 4.6 x 100 mm, 3.5 µm (p/n: 186010643) |
|
Column temperature: |
30 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
3.0 µL |
|
Flow rate: |
1.0 mL/min |
|
Mobile phase A: |
Milli-Q Water |
|
Mobile phase B: |
Acetonitrile |
|
Mobile phase C: |
Methanol |
|
Mobile phase D: |
2% formic acid in water OR 200 mM ammonium hydroxide |
|
Gradient conditions: |
Constant 5% D maintained throughout gradient. Starting conditions of 5% organic (lines B or C), with a linear gradient to 95% organic in 16.43 minutes (6.01% organic per column volume). Hold at 95% organic for 2.76 minutes. Return to 5% organic in 0.02 minutes and hold for 5.51 minutes. Total run time 24.72 minutes. |
Data Management
|
Chromatography software: |
Empower™ 3 Feature Release 4 |
Results and Discussion
The first step of method development, as outlined by the systematic screening protocol, is to test the sample, in this case a mixture of eleven boronic acids, at high and low pH using a C18 stationary phase to assess retention. Given the chemical structures of the compounds, at low pH the analytes should be neutral, while at high pH the acidic boronic acid groups should be at least partially charged. With pKa values of around eight and ten for the two oxygens in the boronic acid group, operating at a pH of approximately ten means that one of the oxygens will be fully charged. However, given that pH is a function of organic modifier present, the actual pH of the separation is probably closer to pH 7, meaning the boronic acid groups will have a partial negative charge. The phenomenon of pH changing when organic modifiers are introduced is a known aspect of liquid chromatography but is often overlooked as it can be complicated.9-10 Figure 2 shows the separation of the eleven boronic acids on an XBridge Premier BEH C18 Column using both low pH and high pH mobile phase additives, formic acid, and ammonium hydroxide, respectively.
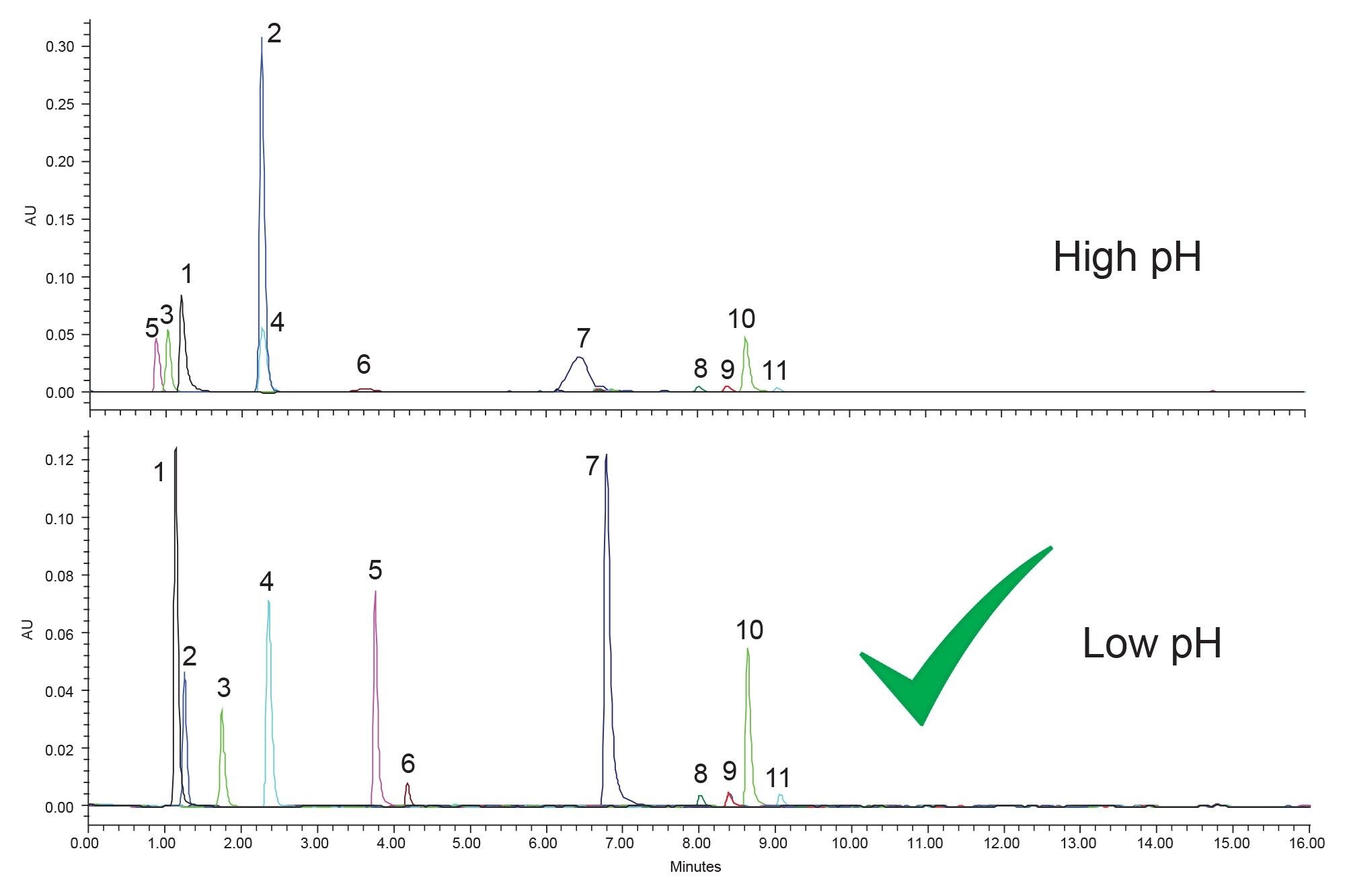
This step of the method development process requires assessment of retention. At high pH some of the boronic acids are less retained than they are at low pH, notably components three and five, which are retained in the void of the column. Component two is better retained at high pH, likely due to the amine group of the compound being neutral. Overall, retention for the compounds is better at low pH as more compounds are retained compared to high pH. The assessment of retention is the only decision that is needed during this step of method development, as subsequent steps will be used to find a suitable separation.
The second step of the systematic screening protocol involves a more typical screening panel, where different column stationary phases are selected and tested using acetonitrile and methanol mobile phases using the previously selected mobile phase additive. For this step, it is important to select diverse stationary phases to screen. Selecting appropriate stationary phases ensures that the results represent a wide range of selectivity while balancing speed of development. Typically, three to four columns are selected, however the number of tested columns is up to the analyst and can sometimes be determined by the instrument configuration.
For this work, in addition to the XBridge Premier BEH C18 Column, four other columns were selected. First, the XSelect Premier CSH C18 Column was selected as it has the same bonded ligand but with a different base particle. The CSH particle is manufactured with a slight positive charge and can provide not only improved peak shape for basic analytes at low pH but can also have slight ion exchange functionality.11
Next, the XBridge Premier BEH Phenyl Column was selected. This column uses a different bonded ligand, specifically designed to interact differently with analytes containing phenyl rings, which is all the boronic acids selected for this application. Next, the XBridge Premier BEH C8 Column was selected as the shorter ligand is less hydrophobic than a typical C18 and can help speed up analysis times. Lastly, the XSelect Premier HSS T3 Column was selected to help retain the early eluting compounds. The HSS T3 stationary phase uses a pure silica base particle, which is different than the two hybrid particles being used in the other phases, and a mid-coverage C18 ligand. By having a lower ligand coverage and the highly retentive silica base particle, the T3 Column is ideally suited to retain more polar analytes. Figure 3 shows the separation of the boronic acids at low pH using methanol mobile phases on the columns indicated.
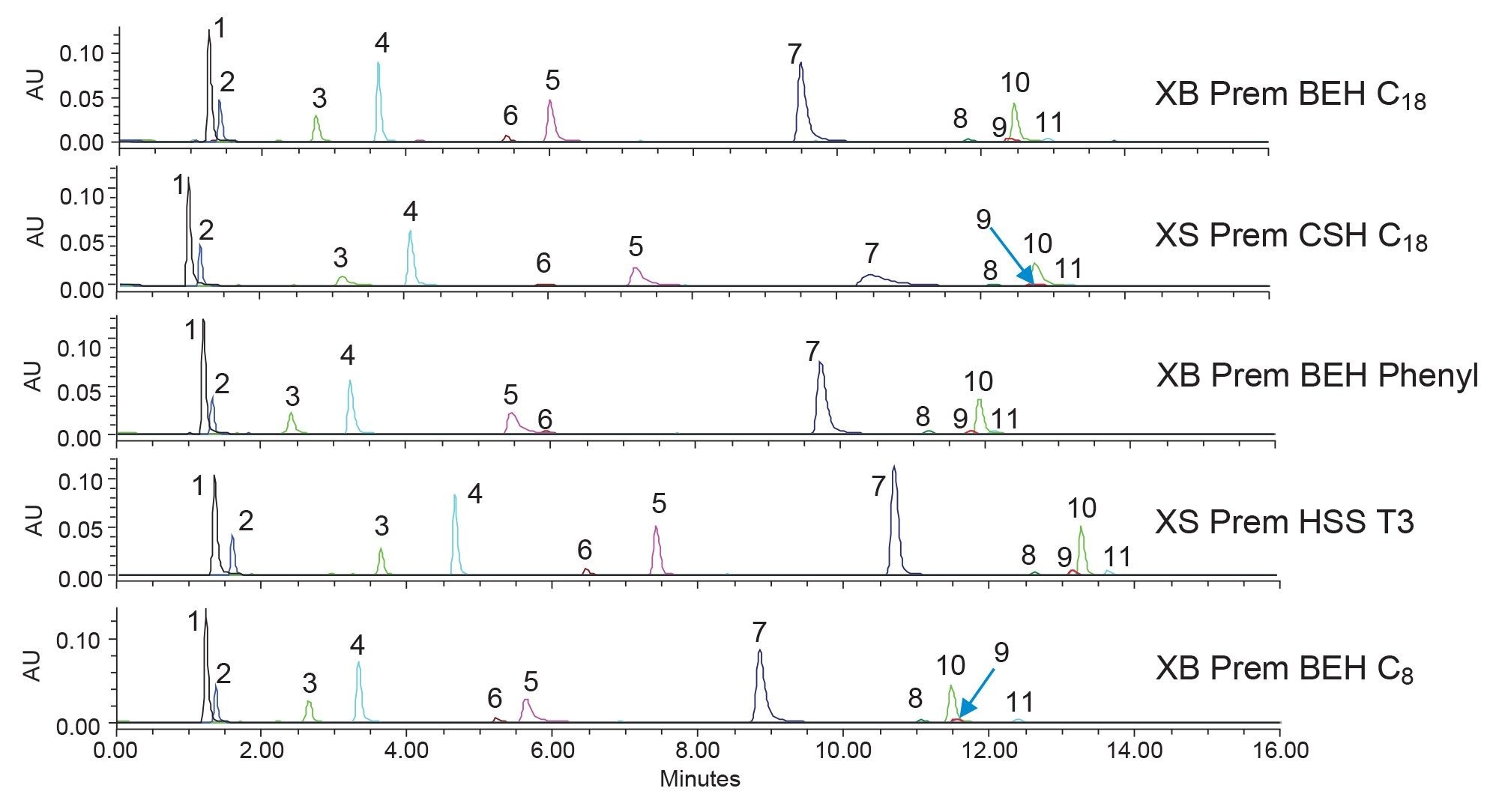
In examination of the screening and attempting to find a suitable separation, several selectivity differences can be seen. First, component nine changes elution order on the XBridge (XB) Premier (Prem) BEH C8 Column, eluting after component ten. On all the other columns, component nine elutes just before or with component ten. Components five and six are also subject to some elution order changes. On most of the columns tested, component five elutes after component six. However, on the XB Prem BEH Phenyl Column, component five is eluted before six. This shift in elution order could be caused by the secondary interactions that can occur with phenyl containing analytes and the phenyl bonded ligand of the stationary phase when methanol is used. While a good range of selectivity and separation is achieved using methanol, none of these are suitable for further use as they all have at least one set of coeluting compounds or poor retention. In addition, peak shape is an issue on some columns, with tailing being a common result. Figure 4 shows the same analytes tested using the same five columns but with acetonitrile used as the strong solvent.
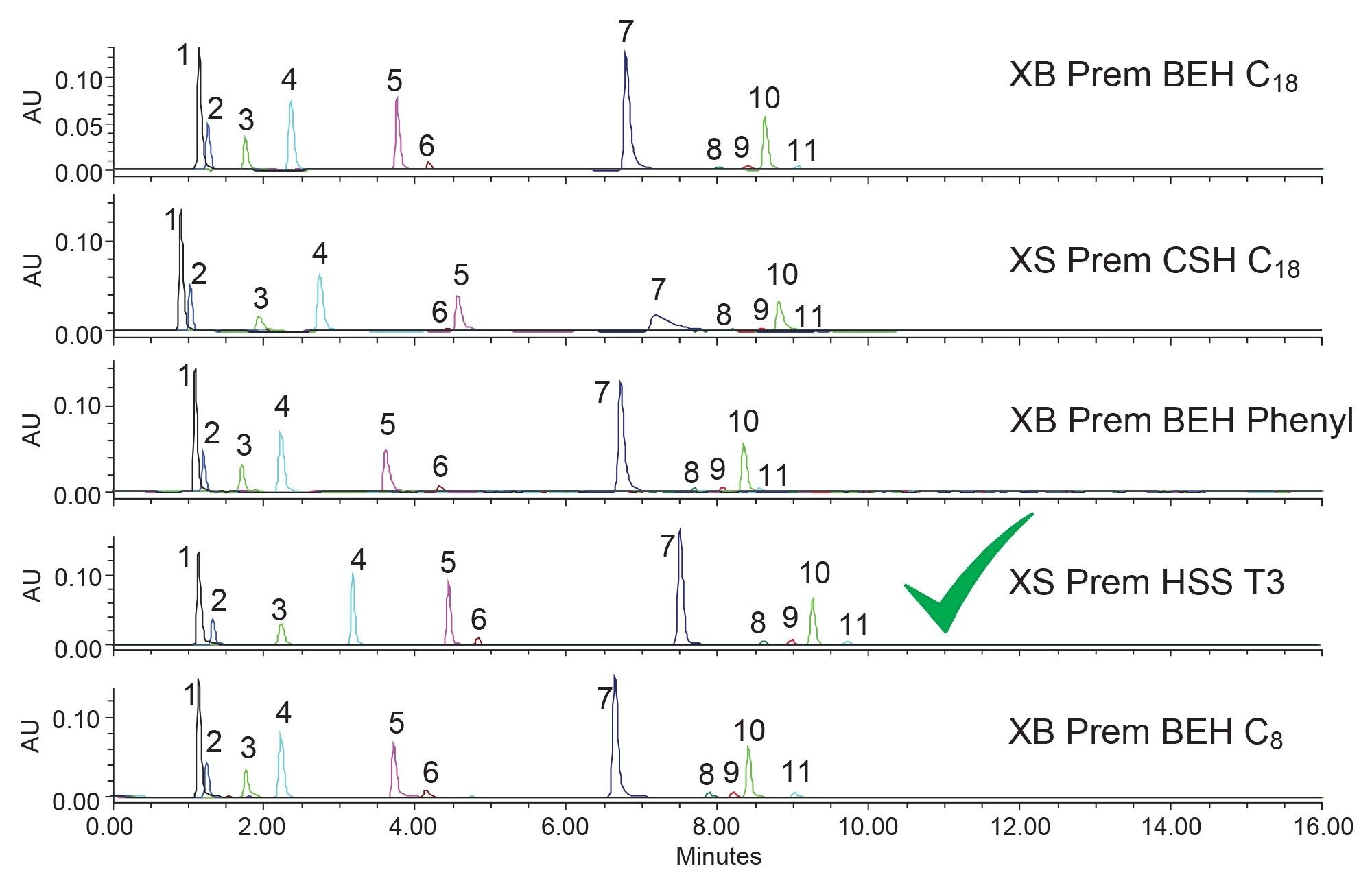
Similar to what was seen with methanol, the five columns produce some selectivity differences when acetonitrile is used. While component nine no longer co-elutes or changes elution order with component ten, components five and six still show some movement across the columns. Notably, the XSelect (XS) Prem CSH C18 column shows that component six elutes before component five with acetonitrile. Component six interacts differently on the CSH particle compared to the BEH or HSS particles used in the other columns tested. This interaction highlights why it is important to screen not only different bonded ligands but also different base particles during method development, as selectivity can be governed by both. For this example, the best separation is obtained using the XSelect Premier HSS T3 Column. While components one and two are not fully resolved, and are not fully retained, the HSS T3 Column does provide the best separation of the two and is therefore the most likely to provide a suitable separation after optimization.
Method optimization can be the most time-consuming step of the systematic screening protocol as it is the least controlled. Optimization is based upon analyst experience as well as other factors like column availability. In this case, the objective of optimization is to increase retention of the probes while improving their resolution. Ideally those two goals can be achieved without sacrificing the performance of the rest of the separation. The first optimization step taken was to begin the gradient at 100% aqueous mobile phase. This should increase the retention of the probes by reducing the mobile phase strength. However, the gradient slope, 6.01% per column volume, must be kept the same to ensure proper separation of the compounds. Not all stationary phases are compatible with 100% aqueous mobile phases as they are susceptible to pore dewetting. However, the HSS T3 stationary phase is compatible due to the lower ligand density differences in particle morphology.12 Figure 5 shows the separation of the boronic acids with the original gradient and then the modified gradient that starts at 100% aqueous.
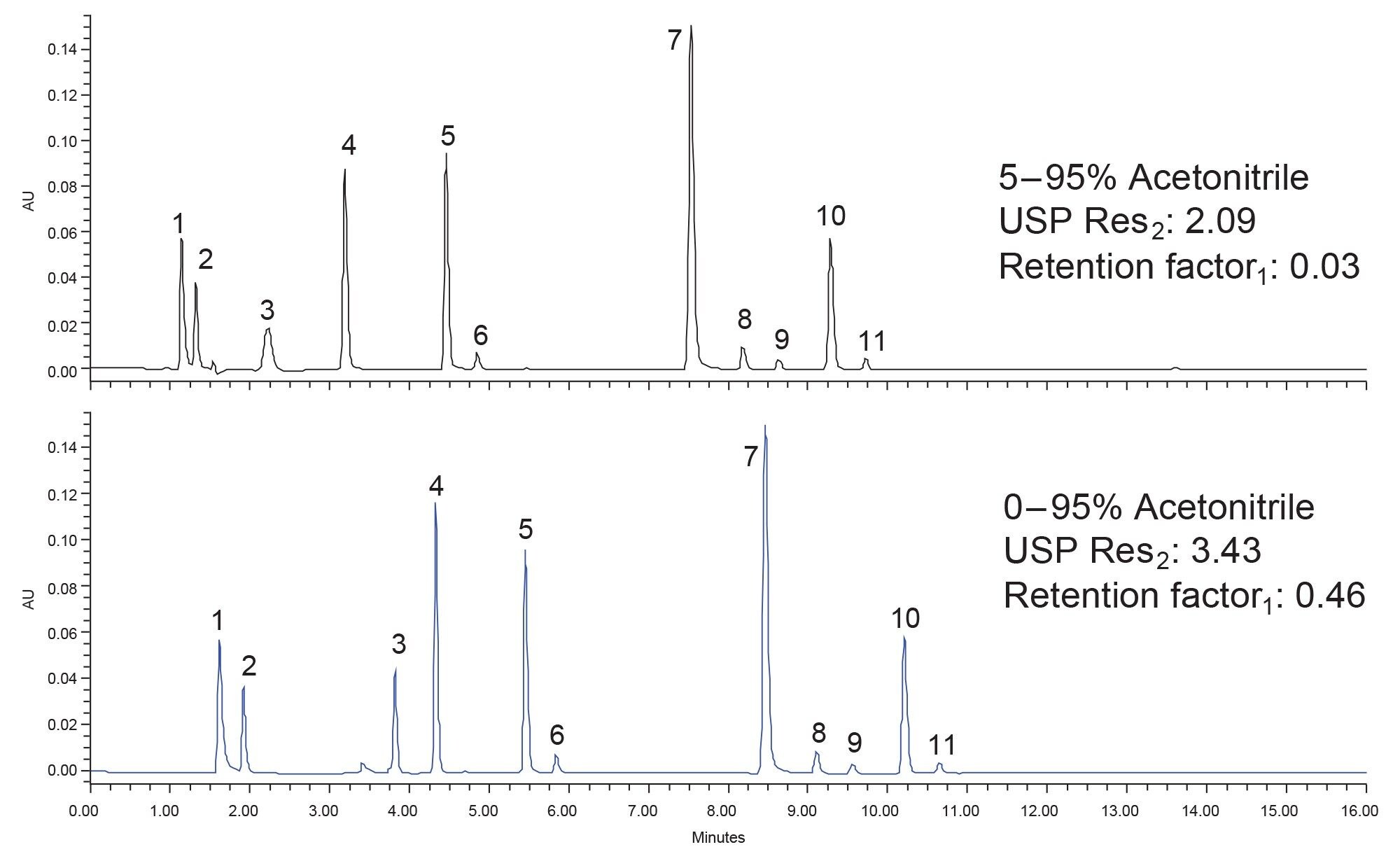
Calculation of USP resolution and retention factor for peak one was performed to determine if the new gradient conditions are better than the original. With an increase in USP resolution from 2.09 to 3.43 and an approximate 15-fold increase in retention factor, the new method conditions are empirically better. While components one and two are still not retained well, they are at least retained outside of the void of the column. Given the properties of those components the retention achieved is acceptable for this application note. With the results obtained, the method development process can stop, and the method can be used for further work, such as tracking reaction progress for mixtures containing one of these boronic acids. This method was developed easily by implementing the systematic screening protocol and employing the versatile MaxPeak Premier columns. By using these two together, an analyst can be certain in the results they are obtaining and can develop their methods faster and more reliably.
Conclusion
Boronic acids are key components in the creation of bioactive molecules. Additionally, they are used in many workflows to “tag” analytes for easier detection and separation. Due to their importance in critical workflows and processes, it is necessary to have a method to analyze these components. Method development for eleven commonly used boronic acids was performed using the systematic screening protocol. This approach streamlines the process of developing new methods by providing a rigid structure and steps to guide the analyst. By combining this approach with MaxPeak Premier columns, which eliminate secondary interactions between analytes and the metal surfaces of the columns, methods can be developed quickly, and reliably. A single method for the separation of eleven boronic acids was developed using an XSelect Premier HSS T3 Column and formic acid modified acetonitrile mobile phases. The method separates all components in under 11 minutes using an Arc HPLC System with PDA detector.
References
- Ertl P, Altmann E, Racine S, Decoret O. Which boronic acids are used most frequently for synthesis of bioactive molecules? Bioorganic and Medicinal Chemistry (2023) 117–405.
- Fossey J, et. Al. The Development of Boronic Acids as Sensors and Separation Tools. The Chemical Record. (2012) 464–478.
- Hong P, McConville P. A Complete Solution to Perform a Systematic Screening Protocol for LC Method Development. Wates White Paper, 720005268EN, 2018.
- Maziarz M, McCarthy S, Wrona M. Improving Effectiveness in Method Development by Using a Systematic Screening Protocol. Waters Application Note. 720005026, 2014.
- Berthelette KD, Turner JE, Walter TH, Haynes K. Using a Systematic Screening Protocol and MaxPeak HPS Technology to Develop a UHPLC Method for the Analysis of Deferoxamine and its Forced Degradation Products. Waters Application note, 720007834, 2022.
- Delano M, Walter TH, Lauber M, Gilar M, Jung MC, Nguyen JM, Boissel C, Patel A, Bates-Harrison A, Wyndham K. Using Hybrid Organic-Inorganic Surface Technology to Mitigate Analyte Interactions with Metal Surfaces in UHPLC. Anal. Chem. 93 (2021) 5773–5781.
- Smith K, Rainville P. Utilization of MaxPeak High Performance Surfaces and the Atlantis Premier BEH C18 AX Column to Increase Sensitivity of LC-MS Analysis. Waters Application Note, 720006745, 2020.
- Walter TH, Alden BA, Belanger J, Berthelette K, Boissel C, DeLano M, Kizekai L, Nguyen JM, Shiner S. Modifying the Metal Surfaces in HPLC Systems and Columns to Prevent Analyte Adsorption and Other Deleterious Effects. LCGC Supplements (2022) 28–34.
- Subirats X, Roses M, Bosch E. On the Effect of Organic Solvent Composition on the pH of Buffered HPLC Mobile Phases and the pKa of Analytes-A Review. Sep & Pur Reviews. (2007) 231–255.
- Walter TH, Alden BA, Berthelette K. Evaluation of the Base Stability of Hydrophilic Interaction Chromatography Columns Packed with Silica or Ethylene-Bridged Hybrid Particles. Separations (2022) 9, 146.
- Kadlecova Z, Kalikova K, Folprechtova D, Tesarova E, Gilar M. Method for evaluation of ionic interactions in liquid chromatography. J Chrom A. (2020) 461–301.
- Gritti F, Walter TH. Retention Loss of Reversed-Phase Columns Using Highly Aqueous Mobile Phases: Fundamentals, Mechanism, and Practical Solutions. LCGC International. (2021) 33–40.
720008307, April 2024