This application extends on Waters technologies for the analysis of intact glycoproteins, glycoprotein subunits, glycopeptides, as well as released N-Linked glycans from biotherapeutic glycoproteins and related compounds.
Sialic acids are a family of nine-carbon acidic monosaccharides that occur naturally at the end of sugar chains attached to the surfaces of cells and soluble proteins.1 A diverse range of sialic acids are found in nature, but the two major sialic acids found on N-glycans and O-glycans in biopharmacuticals are N-acetyl-neuraminic acid (Neu5Ac) and N-glycolyl-neuraminic acid (Neu5Gc). Sialylation can enhance serum half life as well as affecting biological activity such as increasing the anti-inflammatory activity of IgG. Humans cannot synthesize Neu5Gc and its presence on a drug can lead to immune reactions such as chronic inflammation.2 Anti-Neu5Gc antibodies have been detected in normal human sera, and can neutralize any Neu5Gc containing biopharmaceutical lowering its efficacy. The choice of cell line can greatly influence the type of sialic acids present on a biopharmaceutical, for instance 24% of the sialic acids on mouse IgG are Neu5Gc compared to none on human IgG. It is therefore imperative to monitor both the level and types of sialic acids during all stages of the product life cycle. Consequently, ICH guideline Q6B states that the sialic acid content should be determined for glycoprotein biopharmaceuticals since it is considered a critical quality attribute (CQA).3
Among the chromatographic methods there are direct detection methods, such as high-performance anion-exchange chromatography with pulsed amperometric detection (HPAE-PAD),4 and those that require pre-column sample derivatization followed by LC separation with fluorescence detection5 such as that detailed in this application note.
This pre-column derivitization and analyses method begins with the release of the targeted sialic acids from the isolated glycoprotein using mild acid hydrolysis conditions (e.g., 2 M acetic acid for two hours at 80 °C). The freed sialic acid species are then derivitized using 1,2-diamino-4,5-methylenedioxybenzene-2HCl (DMB) dye. The DMB-labeled sialic acids are then separated via reversed-phase (RP) chromatography with subsequent on-line fluorescence detection. Of particular importance to the collection of quality data is the fact that the DMB-labeled sialic acids are light sensitive so samples should be analyzed within 24 hours post labeling to prevent sample degradation that can compromise results. This can become a significant problem if a large number of samples need to be analyzed using traditional HPLC-based techniques that can take more than 30 minutes per sample analysis.
The Glyko Sialic Acid Reference Panel (p/n GKRP-2503) containing Neu5Ac, Neu5Gc, Neu5,7Ac2, Neu5Gc9Ac, Neu5,9Ac2, and Neu5,7(8),9Ac3 was purchased from ProZyme, Inc., 3832 Bay Center Place, Hayward, CA 94545. The DMB labeling protocol is as follows:
|
LC system: |
ACQUITY UPLC System with Fluorescence Detector |
||
|
Columns: |
XBridge BEH C18, 130Å, 3.5 μm, 4.6 x 100 mm (p/n 186003033) XBridge BEH C18, 130Å, XP 2.5 μm, 3.0 x 75 mm (p/n 186006034) ACQUITY UPLC BEH C18, 130Å, 1.7 μm, 2.1 x 50 mm (p/n 186002350) |
||
|
Column temp.: |
30 °C |
||
|
Sample temp.: |
4 °C |
||
|
Mobile phase: |
Acetonitrile:Methanol:Water (9%:7%:84%) |
||
|
Strong and weak needle wash: |
Acetonitrile:Methanol:Water (9%:7%:84%) |
||
|
FLR detection: |
Excitation wavelength: 373 nm, Emission wavelength: 448 nm |
||
|
Column configuration: |
2.1 x 50 mm |
3.0 x 75 mm |
4.6 x 100 mm |
|
Flow rate: |
0.18 mL/min |
0.26 mL/min |
0.43 mL/min |
|
Injection volume: |
0.7 μL |
2.0 μL |
6.7 μL |
|
Data management: |
Empower Pro (v3) Software |
The Waters Application Note entitled: “Future Proofing the Biopharmaceutical QC Laboratory: Chromatographic Scaling of HPLC Monosaccharide Analyses Using the ACQUITY UPLC H-Class Bio System” (p/n 720005255EN) details the steps and considerations necessary to successfully develop a HPLC-, UHPLC-, or UPLC-based separation of hydrolyzed monosaccharides derived from glycoproteins. This application note details the application of the same scaling principles to the LC-based analysis of DMB-labeled sialic acids on the same Waters BEH C18, 130Å column chemistry.
A series of experiments were performed using the XBridge BEH C18, 130Å, 3.5 μm, 4.6 x 100 mm Column to determine whether a gradient or an isocratic separation could be developed to yield adequate DMB-labeled, sialic acid reference standard component resolution. Results from this work confirmed that an eluent containing acetonitrile:methanol:water (9%:7%:84%) could be used to yield baseline resolution of the sialic acid species of interest without the need to run a gradient. While the shown HPLC-based column separation (Figure 1, top) would adequately address many laboratory sample throughput needs, the next series of experiments were undertaken to demonstrate that the same high-quality separation could be performed using the same BEH C18, 130Å-based chemistry but of reduced particle size and column I.D. and length for those requiring higher sample throughput (Figure 1, middle and bottom) using the scaling techniques detailed in Waters Application Note p/n 720005255EN.
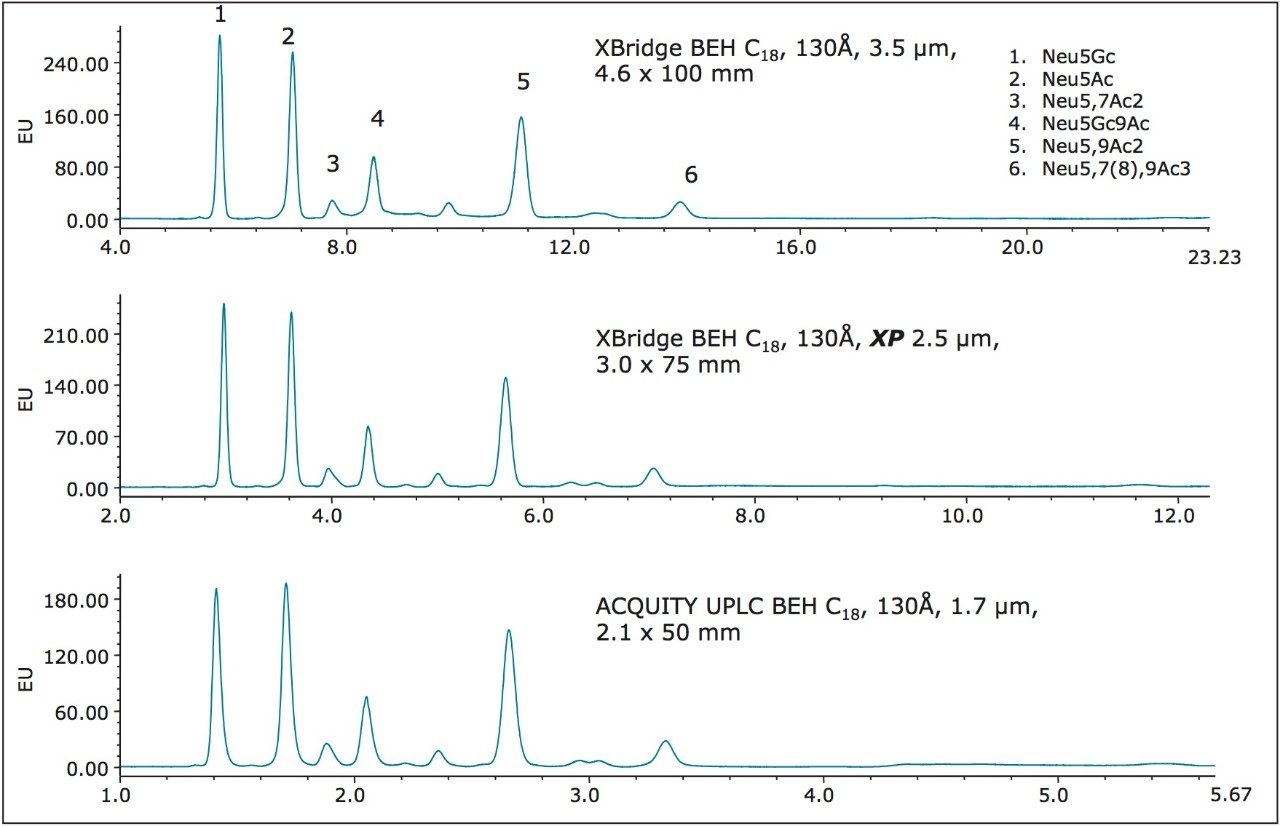
This application note clearly demonstrates the ability to obtain high-quality, DMB-labeled sialic acid analyses on geometrically scaled HPLC, UHPLC, and UPLC column configurations containing same Waters BEH C18, 130Å reversed-phase-based chemistry on 3.5 μm (HPLC), 2.5 μm (UHPLC), and 1.7 μm (UPLC) particles. For high sample throughput needs, the UPLC column and separation conditions provide adequate component resolution with an approximate four-fold reduction in analysis time and eluent consumption.
720005550, December 2016