This application note details about isolation of impurities in drugs using SunFire Columns.
SunFire C18 analytical and OBD preparative columns are designed to provide maximum loadability in simple mobile-phase conditions, accurate scalability, and high peak capacity. They are ideal for isolating impurities in crude drug samples.
Nimodipine is a calcium-channel blocker, which acts on blood and heart vessels. Several impurities may be formed during its synthesis. The existence of impurities even in small amounts may influence the efficacy and safety of drugs. Identification of impurities is important to get insight of pharmaceuticals. Advanced analytical technologies, such as HPLC and MS-MS, are efficient ways to detection those trace impurities.
SunFire C18 Columns are engineered with highly pure raw materials and a tightly controlled synthesis process. These columns provide high efficiencies, maximum loading, and symmetric peak shapes for the analysis of acids, neutrals, and bases. SunFire C18 preparative columns are manufactured with the Optimum Bed Density (OBD) design to deliver consistent column-to-column performance, unmatched column lifetime with DMSO sample diluents, and accurate scalability.
|
Columns: |
SunFire C18 4.6 x 100 mm, 5 μm and 19 x 100 mm, 5μm |
|
Mobile phase A: |
0.1% formic acid in water |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
Flow rate: |
1.4 mL/min analytical, 23.9 mL/min preparative |
|
Analytical gradient: |
10-min linear from 30% to 90% B, with 1 min initial hold time |
|
Preparative gradient: |
10-min linear from 30% to 90% B with 1.97 min initial hold time |
|
Injection volume: |
200 µL (analytical) and 3400 µL (preparative) |
|
Sample: |
Crude nimodipine prepared in DMSO at 30 mg/mL |
|
Total mass loading: |
6 mg (analytical), 102 mg (preparative) |
|
Detection: |
UV at 290 nm |
|
Instrument: |
Waters AutoPurification System |
|
Interface: |
Positive-mode ESI |
|
Capillary: |
3.5 kV |
|
Cone: |
30 V |
|
Source temperature: |
150 °C |
|
Desolvation temperature: |
250 °C |
|
Cone gas flow: |
50 L/h |
|
Desolvation gas flow: |
250 L/h |
|
Instrument: |
Waters Micromass Quattro micro API |
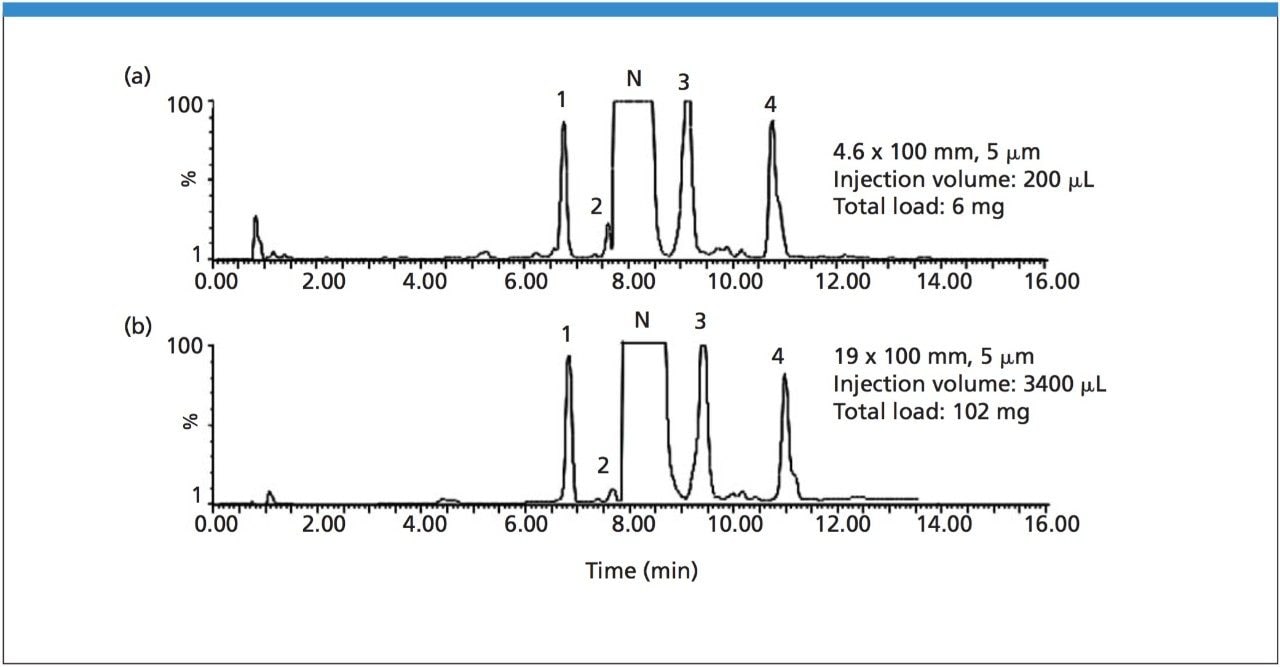
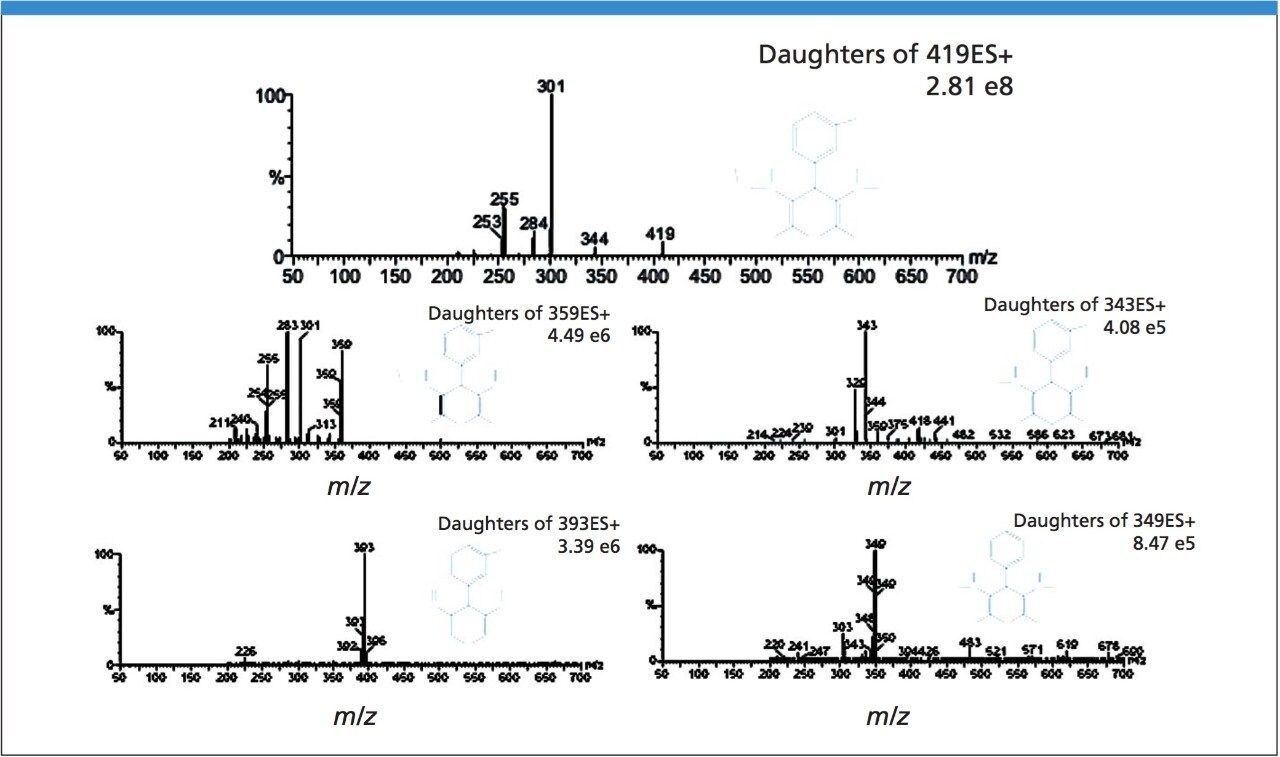
The separation of nimodipine and four impurities on the analytical column is shown in Figure 1A. The total load is 6 mg. The chromatograms are baseline enhanced in order to identify the impurity peaks. As shown in the figure, closely eluting impurity 2 has been detected and separated from nimodipine. The separation was proportionally scaled-up and run on the preparative column as shown in Figure 1B. Note the direct scale up, excellent peak shapes, and total mass load of 102 mg. Each peak in Figure 1A was collected in separate vials first, and then analyzed using MS-MS. Nimodipine has the molecular weight of 418.1 (peak N). The daughter ion scan of 419 was shown on Figure 2, top one. Similarly, the daughter ion scan was applied to get further structural information on peaks 1, 2, 3, and 4. Finally, we determined the structures based on the MS-MS data as shown in Figure 2.
Highly efficient isolation and direct scale-up are observed on SunFire C18 analytical and preparative columns. This column technology ensures rapid target purifications with minimal chromatographic development. The combination of Waters’ chemistry and triple-quadrupole mass spectrometers is a powerful tool for drug impurity profiling.
WA41939, June 2005