
In this application note, linalool and terpinen-4-ol were used to demonstrate the chiral purification capabilities of SFC for volatile flavor and fragrance compounds. First, a recovery study was performed by collecting the enantiomers from (±)-terpinen-4-ol and (±)-linalool standards. These compounds were then enantiomerically purified from lavender and tea tree essential oils. Stacked injections and fast chiral separations reduced the time required for the enantiomeric purification of flavor and fragrance compounds.
Found in foods, wines, spices, perfumes, and essential oils, flavors and fragrances are abundant in nature and enhance life.1 In general, these compounds are small molecules that are sufficiently volatile to be sensed through taste or smell. They cover a wide range of chemicals, including terpenes, phenols, aldehydes, and esters, many of which are chiral.2 By obtaining them through natural sources, chemical synthesis, or fermentation, the flavor and fragrance industry ensures a constant infusion of new experiences to surprise and please the chemical senses.1-2
The interaction of compounds with biological systems has long been shown to be stereoselective.3 Just as enantiomers of chiral drugs exhibit different pharmacological activity, chirality also plays an important role in the flavor and fragrance chemistry.4 These chiral enantiomers dictate not only taste or odor quality, but also intensity. Therefore, chemists devote great effort to investigate enantiopure chiral flavors and fragrances.5 Care is taken to make sure only the desired odor or flavor active isomer is added to reduce toxicological risks and, specifically, to meet strict regulations in the food and beverage industries. To that end, highly pure compounds are required, and no toxic chemicals are permitted during preparation.2
Many flavor and fragrance compounds are purified by simple fractional distillation. However, this technique does not have the same selectivity as chromatographic methods, nor can it distinguish between chiral enantiomers. Currently, the most common method for purifying flavor and fragrance compounds is by prep GC. While this technique exhibits high resolution for these types of volatile compounds, purification by GC can be quite challenging. GC exhibits low loading capacities due to the limitations of capillary columns, as well as long run times, often resulting in only a few micrograms being collected over several hours. Also, the high temperatures typical of GC increase the risk of sample loss and degradation. To enable fraction collection by GC, cooled (often with liquid nitrogen) traps are required, and multiple traps are needed if there is more than one compound of interest in a sample.6
SFC is a chromatographic technique that employs compressed CO2 as the main component of the mobile phase. In contrast to GC, packed columns and low temperatures improve column loading and compound stability. A simple multiport valve (for easy collection of multiple peaks) and a make-up pump simplify fraction collection without the need for special trapping equipment.
In this application note, linalool and terpinen-4-ol (Figure 1) were used to demonstrate the chiral purification capabilities of SFC for volatile flavor and fragrance compounds. First, a recovery study was performed by collecting the enantiomers from (±)-terpinen-4-ol and (±)-linalool standards. These compounds were then enantiomerically purified from lavender and tea tree essential oils. Stacked injections and fast chiral separations reduced the time required for the enantiomeric purification of flavor and fragrance compounds.
All recovery and method development samples were made up at concentrations of approximately 10 mg/mL in ethanol. Collections were performed in sets of ten 100-μL stacked injections, for a total of 1mL injected. The recoveries were performed in triplicate and all collected enantiomeric fractions were transferred to 25-mL volumetric flasks and brought up to volume in ethanol. Re-analysis standards were made by diluting 1 mL (the total injection volume) of the 10-mg/mL solutions in 25 mL ethanol. Due to the fairly small percentage of the desired compound in the tea tree and lavender essential oils, these samples ultimately required higher concentrations of 50 mg/mL and 30 mg/mL, respectively.
|
SFC system: |
Waters Investigator SFC System |
|
Preparative column: |
CHIRALPAK AD-H, 5 μm, 10 x 250 mm |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
Ethanol |
|
Make-up solvent: |
Ethanol |
|
Flow rate: |
12 mL/min |
|
Additional conditions: |
Recorded in Table 1 |
Fraction analysis was performed on the same Investigator SFC system, using a 5 μm, 4.6 x 250 mm CHIRALPAK AD-H Column. For the recovery study, the areas for the diluted enantiomeric fractions were compared against the areas for the re-analysis standards. The undiluted fractions from the essential oils were tested for %purity against all impurities (enantiomeric or matrix).
|
Detector: |
2998 PDA Detector |
|
Scan: |
220–300 nm |
|
Collection: |
Single wavelength 220 nm |
ChromScope v1.2
There are many challenges inherent in the chiral purification of volatile flavor and fragrance compounds. The first challenge is in the chromatography itself; not only do the compounds have to be resolved achirally from the matrix, but enantiomeric resolution is also required. For purification, good chromatographic resolution enables higher column loading, improved product purity, and efficiency. Because of the volatile and instable nature of these compounds, analysis and purification must be accomplished without significant sample loss due to evaporation or degradation. Finally, the preparation of these compounds requires the use of non-toxic chemicals, especially if the products are destined for human use or consumption.
SFC uses CO2 as the primary component of the mobile phase, and in most cases alcohol as solvent B. In this case, ethanol is used due to its non-toxic nature and compatibility with not only flavor and fragrance analytes, but also with any post purification processes down-stream. Also, due to the evaporation of the CO2 after collection, there is much less solvent collected than in traditional LC techniques. Another advantage of SFC for the purification of flavor and fragrance compounds is the relatively mild conditions used for separation compared to GC or HPLC. Low temperatures and lack of additives decrease the possibility of evaporation and degradation of the target compounds during analysis and purification.
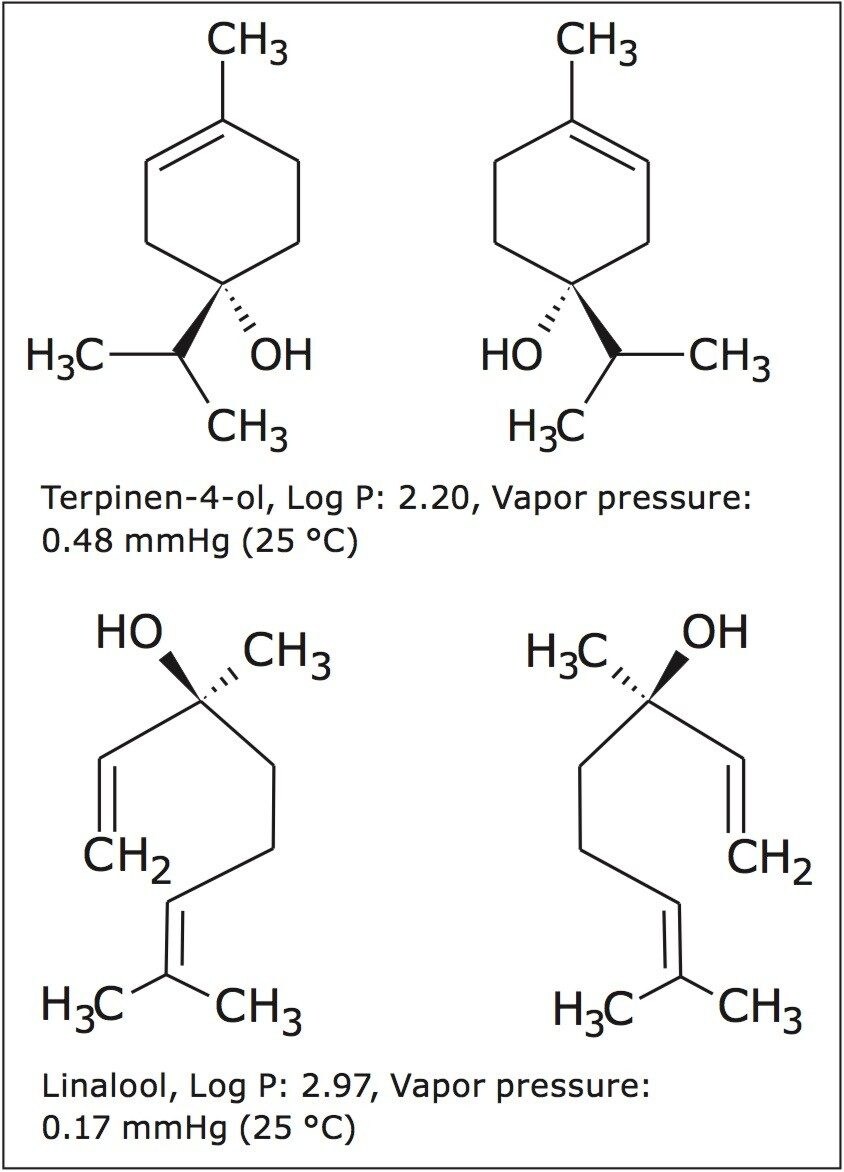
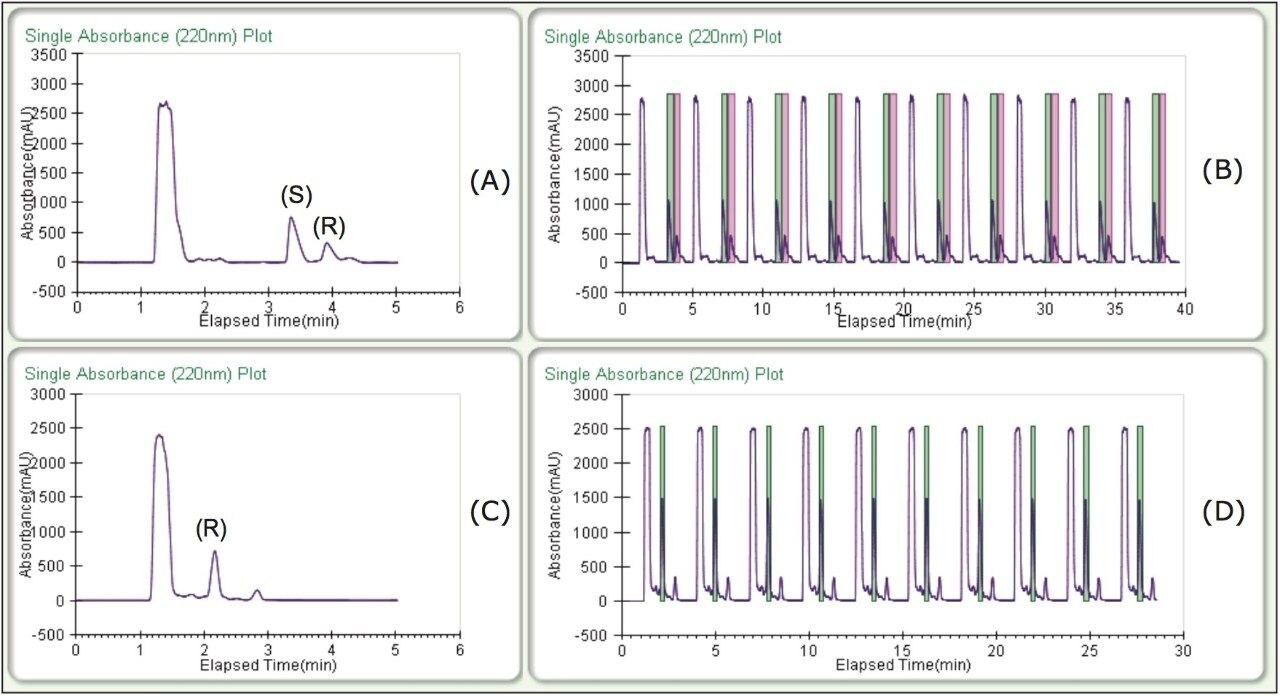
Enantiomeric separations of the linalool and terpinen-4-ol isomers were achieved under isocratic conditions, on the 5 μm, 10 x 250 mm CHIRALPAK AD-H Column in less than 4 minutes. High resolution and good peak shape allowed for loading of 1 mg per injection. Based on this chromatography, much more could have been loaded, but for the purposes of the recovery study, this loading was ideal. Isocratic method conditions made it possible to do stacked injections, resulting in the injection and collection of approximately 10 mg in less than 30 minutes (figure 2) for both terpinen-4-ol and linalool chiral purifications.
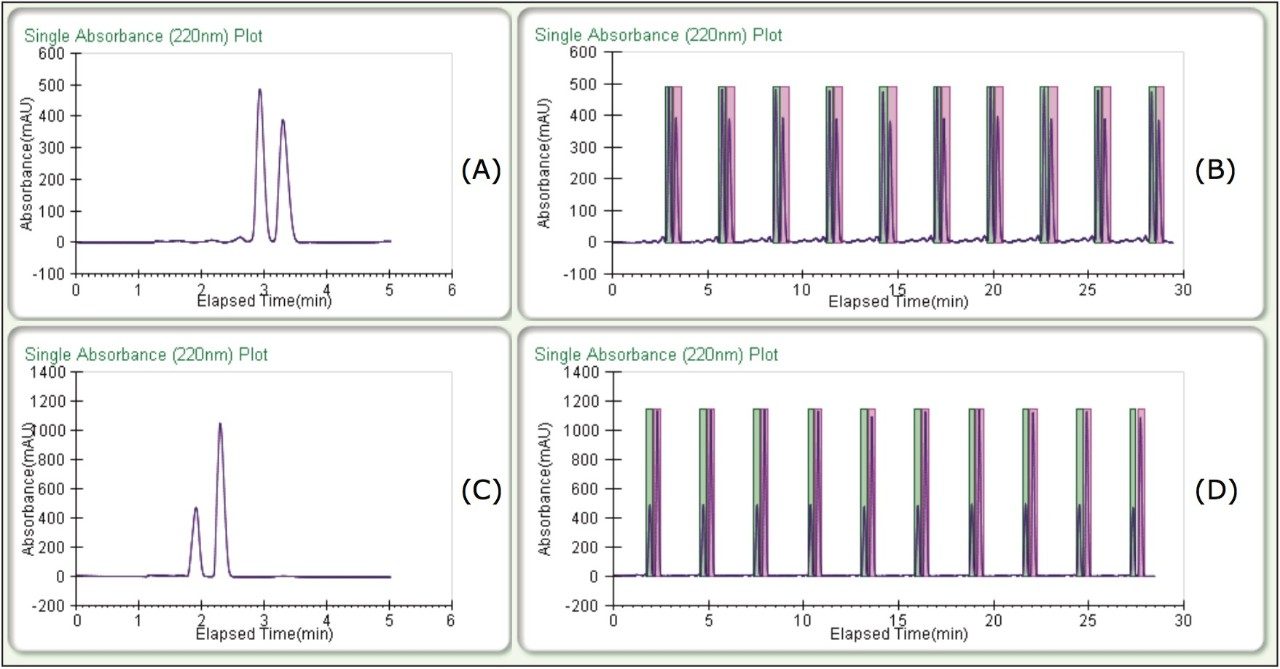
As part of the recovery study, different heat exchanger (HE) temperatures were tested to optimize recovery. The terpinen-4-ol recovery was unaffected by temperature and collection was carried out at an HE temperature of 35 °C. The linalool recovery, however, was optimized at a lower HE temperature of 25 °C. Percent recoveries at the optimized conditions (average yields are in the table) and chromatograms of the fractions can be viewed in Figure 3. Three sets of stacked collections (ten 100-μL injections) were done for each compound under the optimized conditions. Based on the volatile nature of these compounds and the fairly low recoveries typically reported, 70–80% recovery was notable. Also, all fractions in the recovery study exhibited >99% enantiomeric purity.
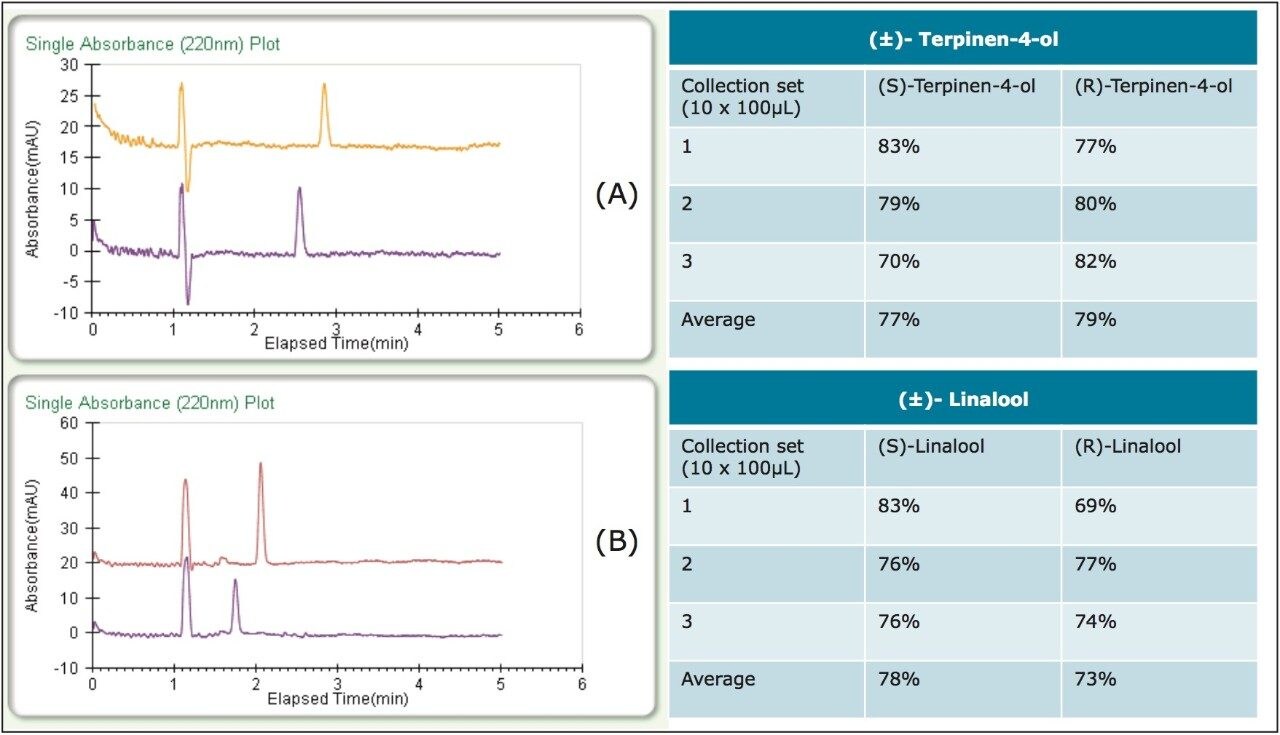
To test the purification technique with real samples, (R)- and (S)-terpinen-4-ol and (R)-linalool were purified from tea tree and lavender essential oils, respectively. Some method development was necessary to separate the enantiomers from their matrices, and to improve loading. The resulting methods were isocratic, allowing for stacked injections, and increasing collection efficiency. Due to the lower %content in the essential oils as compared to the standards, much higher loading was needed to get an acceptable yield for the compounds of interest. As a result, the samples were made up at much higher concentrations, 50 mg/mL for the tea tree oil, and 30 mg/mL for the lavender oil.
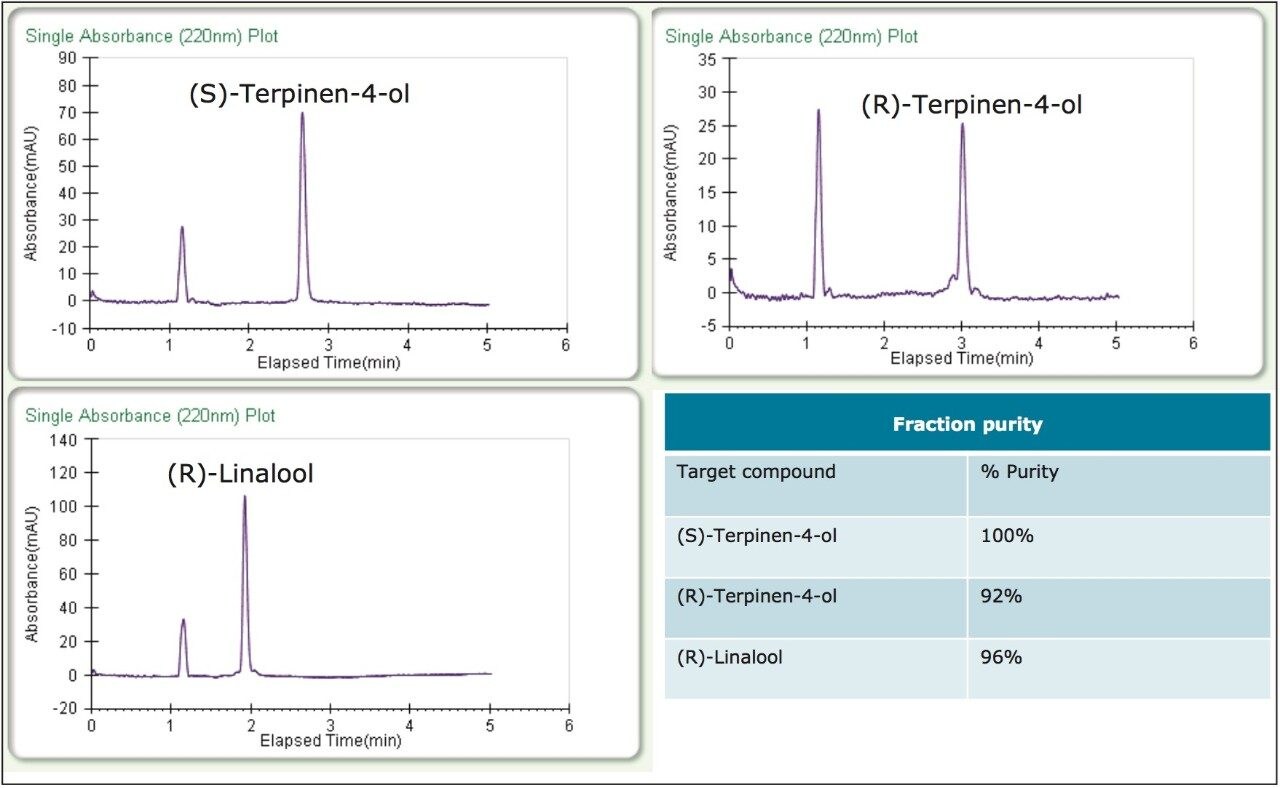
Figure 4 shows the separations and collection for the two essential oil purifications. The collected fractions were re injected directly without dilution; the resulting purity analysis can be seen in figure 5. The three target peaks were isolated resulting in >99% enantiomeric purity, and the overall chemical purity was also quite good (>92%).
720005150, August 2014