The ionKey/MS System consisting of the ACQUITY UPLC M-Class System, ionKey Source, and the Xevo G2-XS QTof provides high sensitivity for biotherapeutic applications. Data from this configuration can be processed in UNIFI Scientific Information System to provide an automated solution that reduces human error in data workup.
Results from an intact IgG1 mAb mass analysis were used to illustrate the sensitivity capabilities that can help biopharmaceutical laboratories extend precious samples, and detect low level glycoforms.
LC-MS has emerged as a powerful and robust tool for the characterization of intact proteins. This approach has been widely applied to heterogeneous therapeutic monoclonal antibodies, antibody drug conjugates, bispecific mAb, antibodyantigen complexes, and antibody mixtures, to name a few. The ability to also separate the proteins from impurities, small molecules, and dissociated light and heavy chains, allows for cleaner spectra with less ion adducts. It also allows for the ability to discern glycoform variants, post translational modifications, and genetic modifications, for example. The data from such experiments is a critical component to the biopharmaceutical drug development process.
The ionKey/MS System offers a number of advantages for therapeutic monoclonal antibody analysis, including increased ESI efficiency leading to improved MS sensitivity, reduced sample overheads, and reduced solvent consumption for high throughput analysis. The iKey Separation Device contains the fluidic connections, electronics, ESI interface, column heater, eCord, and chemistry to perform UPLC separations in the source of the mass spectrometer. It provides ease-of-use that has historically not been present with microflow LC-MS systems. Specifically, integrated microfluidics combined with clamp-on connections allows for zero dead volume connections in seconds. Alternative chemistries and maintenance can be performed rapidly with minimal system downtime. In addition, integrated heating elements, memory, and ESI tips provide easy programming, control of LC-gradients and ESI spray in a customized environment.
The ionKey/MS System consisting of the ACQUITY UPLC M-Class System, ionKey Source, and the Xevo G2-XS QTof provides high sensitivity for biotherapeutic applications. Data from this configuration can be processed in UNIFI Scientific Information System to provide an automated solution that reduces human error in data workup.
Results from an intact IgG1 mAb mass analysis were used to illustrate the sensitivity capabilities that can help biopharmaceutical laboratories extend precious samples, and detect low level glycoforms.
|
UPLC conditions |
|
|
LC system: |
ACQUITY UPLC M-Class System |
|
Separation device: |
iKey Protein BEH C4 Separation Device, 300Å, 1.7 μm, 150 μm x 50 mm (p/n 186006765) |
|
iKey temp.: |
80 °C |
|
Loop size: |
1 μL |
|
Injection volume: |
Full loop mode |
|
Flow rate: |
5.0 μL/min |
|
Mobile phase A: |
Water with 0.1% formic acid |
|
Mobile phase B: |
Acetonitrile with 0.1% formic acid |
|
Weak needle wash: |
Water with 0.1% formic acid |
|
Strong needle wash: |
50% acetonitrile, 25% methanol, 25% water |
|
Seal wash: |
90:10 water:acetonitrile |
|
Time(min) |
Flow(μL/min) |
%A |
%B |
Curve |
|---|---|---|---|---|
|
Initial |
5.0 |
97.0 |
3.0 |
Initial |
|
1.0 |
5.0 |
97.0 |
3.0 |
6 |
|
4.5 |
5.0 |
3.0 |
97.0 |
6 |
|
9.0 |
5.0 |
3.0 |
97.0 |
6 |
|
9.5 |
5.0 |
97.0 |
3.0 |
6 |
|
13.0 |
5.0 |
97.0 |
3.0 |
6 |
|
MS system: |
Xevo G2-XS QTof with ionKey/MS |
|
Ionization mode: |
ESI + |
|
Capillary voltage: |
3.5 kV |
|
Source temp.: |
150 °C |
|
Cone voltage: |
190 V |
|
Source offset: |
150 V |
|
Cone gas: |
50 L/h |
|
Nano Flow gas: |
0.10 Bar |
|
Quad profile Scan: |
Auto 500–4,000 Da, 1 second |
|
Data format: |
Continuum |
|
Analyzer mode: |
Sensitivity |
|
RF settings: |
RF Amplitude: Collision 400 V Gain 10 V |
A Waters glycosylated Intact mAb Mass Check Standard (p/n 186006552) was constituted in 3% acetonitrile and 0.1% formic acid, sonicated for 5 minutes, and vortexed prior to insertion into the sample manager at 10 °C. New samples were prepared daily.
The Intact mAb Mass Check Standard was serially diluted and injected with the on-column mass noted in Figure 1, followed by a blank. The results illustrate an integrated total ion chromatogram (TIC), and show the total charge envelope of the mAb (left) and a single charge state (52+) selected from this envelope (right). Glycoform variants were clearly detected down to 1 ng (on-column) including (G0F/G0F, G0F/G1F, G1F/G1F, G1F/G2F, and G2F/G2F).
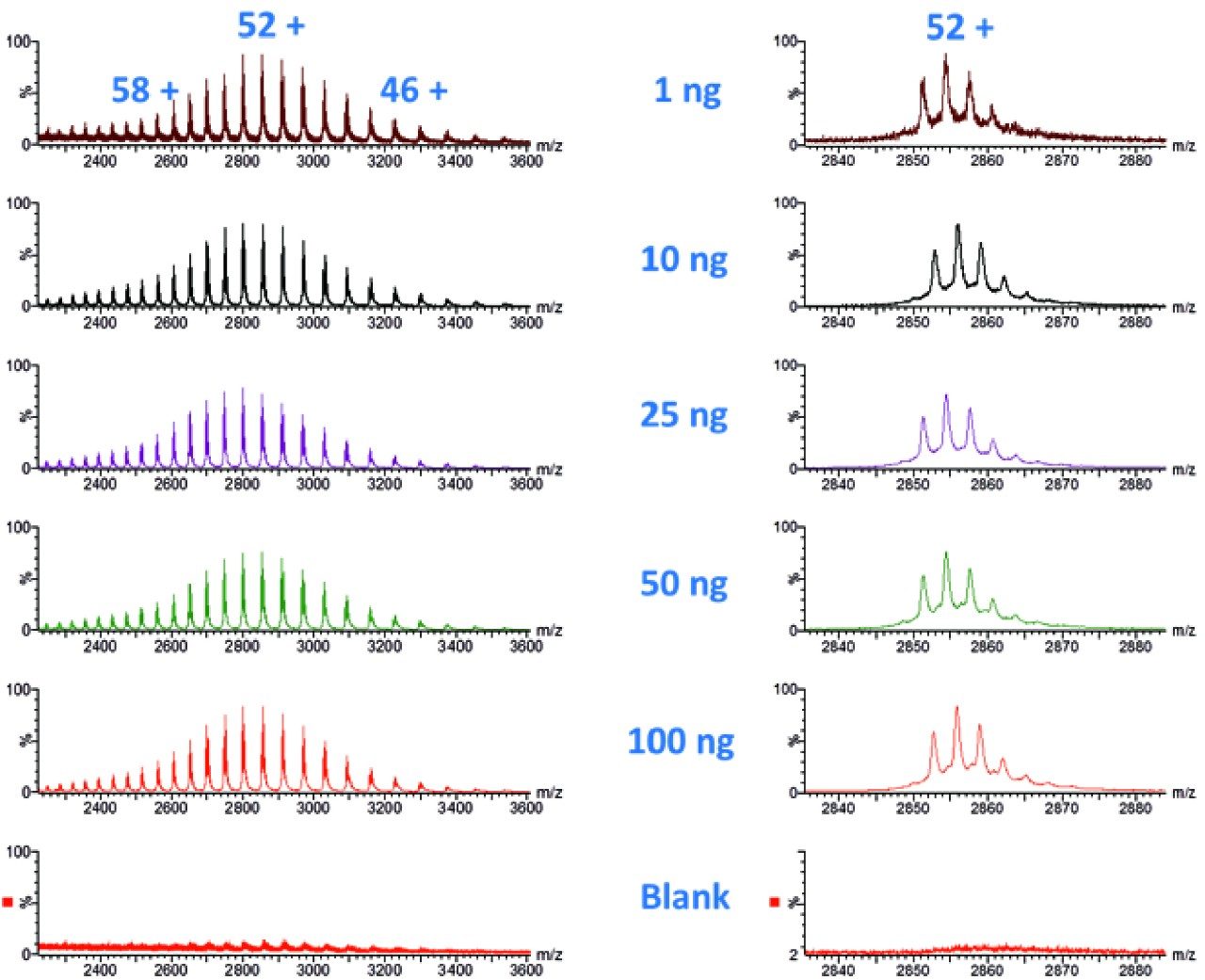
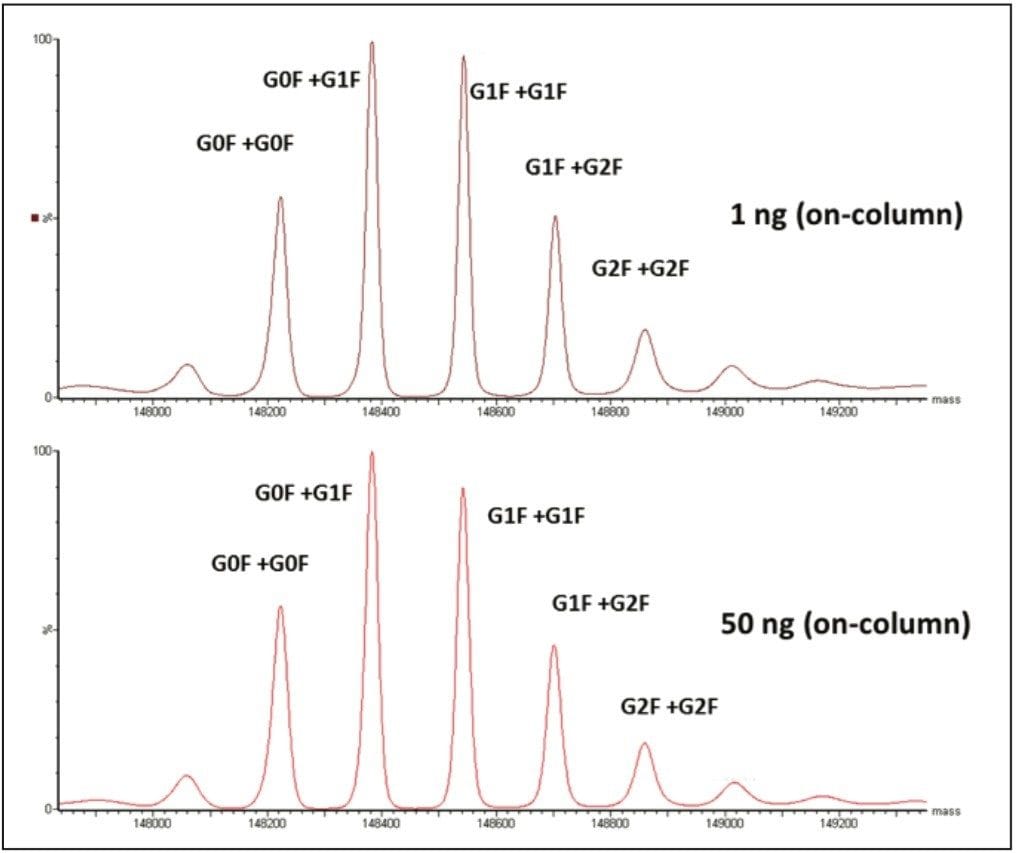
Figure 2 demonstrates a deconvoluted spectrum from both the 1 and 50 ng (on-column) loads. Deconvoluted spectrum was a result of TIC integration followed by a MaxEnt deconvoluted MS spectrum that corresponds to the summed spectra under the detected peak. The spectrum was within 2 ppm, illustrating no significant deterioration of mass accuracy at the 1 ng detection limit of the Waters mass check mAb standard. Accurate mass of the glycoforms were achievable between 1–100 ng on-column.
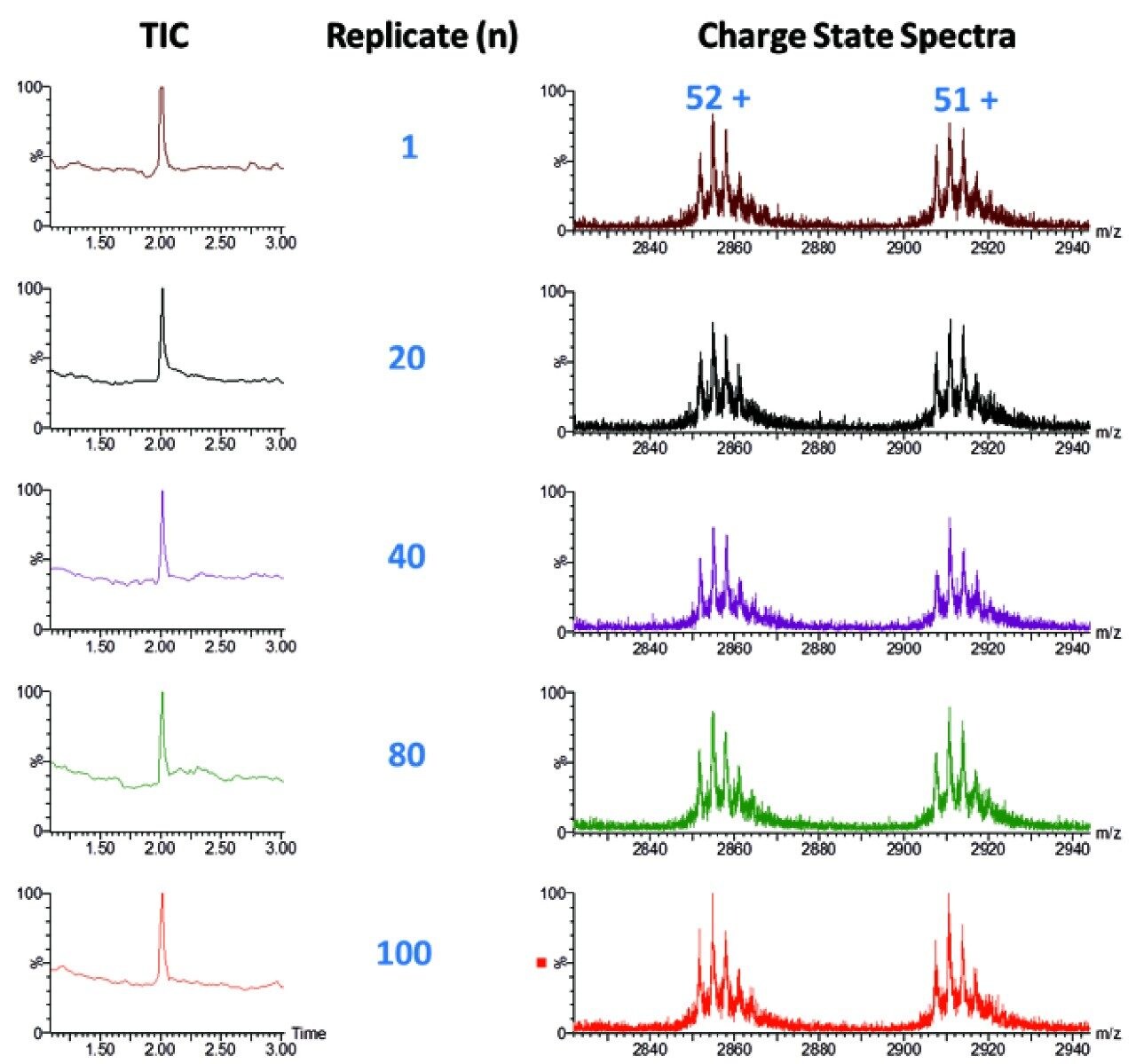
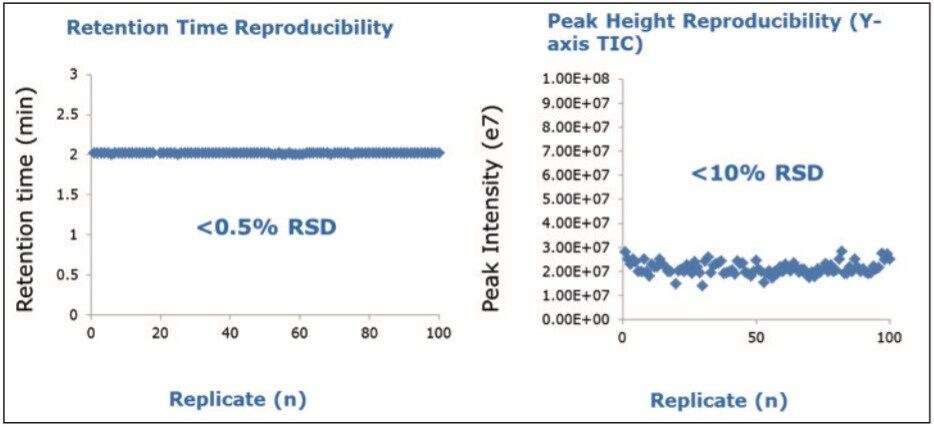
Reproducibility studies were performed over a 100 replicate injections for the Waters standard. Figure 3 demonstrates the 100 replicate for the Waters standard with 1 ng on-column loading. Glycoform variants were robustly measured over this range with good retention time reproducibility and peak height reproducibility, as shown in Figure 4.
720005378, April 2015