
An important property of a vial cap is the ability to maintain the vial analyte concentration after multiple injections over time. This ability is influenced by different factors such as the volatility of the analyte/diluent, the number of injections, the elapsed time and the injector/cap septum used. The results of this study can provide guidance in this area.
Understand the different factors that affect vial analyte concentration when repeated injections over long time periods are performed.
When selecting a vial, cap and septum to contain analytical solutions, there are several factors to take into account. In most analyses, the cleanliness of the vial and the septum are most important.1,2 With analyses where multiple injections of the same analyte(s) are performed over time, the ability of the septum to reseal is a consideration. For example, to analyze a system suitability standard or reference material at regular intervals, it is useful to be able to use the same vial and cap and know that the septum will reseal sufficiently to prevent significant changes in analyte concentration due to evaporation.
This application note will examine the effect of sample vial cap type on analyte concentration once the cap septum is punctured. The study used two separate LC instruments which have two different needle assemblies: a Waters ACQUITY UPLC with a fixed loop injector and a Waters ACQUITY UPLC H-Class with a flow through needle injector (FTN). The Reversed Phase QC Reference Material (QCRM) was used in this study as it contains both volatile and non-volatile compounds and it is made in an aqueous solution of a volatile solvent, typical of many analytical samples.
|
Mobile phase A: |
0.1% formic acid in water |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
Gradient: |
5-95% B in 2.25 min, hold for 0.25 min, return to 5% B in 0.1 min, hold for 1.4 min. total run time: 4 minutes |
|
Flow rate: |
0.6 mL/min |
|
Column: |
ACQUITY CSH C18, 1.7 μm, 2.1 x 50 mm (p/n 186005296) |
|
Column temperature: |
30° C |
|
Sample temperature: |
10° C |
|
Detection: |
UV @ 254 nm |
|
Injection volume: |
1.0 μL |
|
Data Management: |
Empower 3 CDS |
Ampoules of the Reversed Phase QCRM (p/n: 186006363) were used. Each ampoule was opened and a 200 uL aliquot was transferred into each LCGC Certified Qsert Vial (p/n: 186002804). The vials were immediately closed with the following Waters cap types (screw caps with PTFE/silicon septa):
TruView Pre-Split (p/n 186005827)
TruView Solid (p/n 186005826)
Blue LectraBond Pre-Split (p/n 186000305)
Blue LectraBond Solid (p/n 186000274)
LCMS Pre-Split (p/n 186005829)
LCMS Solid (p/n 186005828)
The QCRM contains the following compounds at the described concentration in 65:35 methanol: aqueous 20mM potassium phosphate buffer at pH 7.
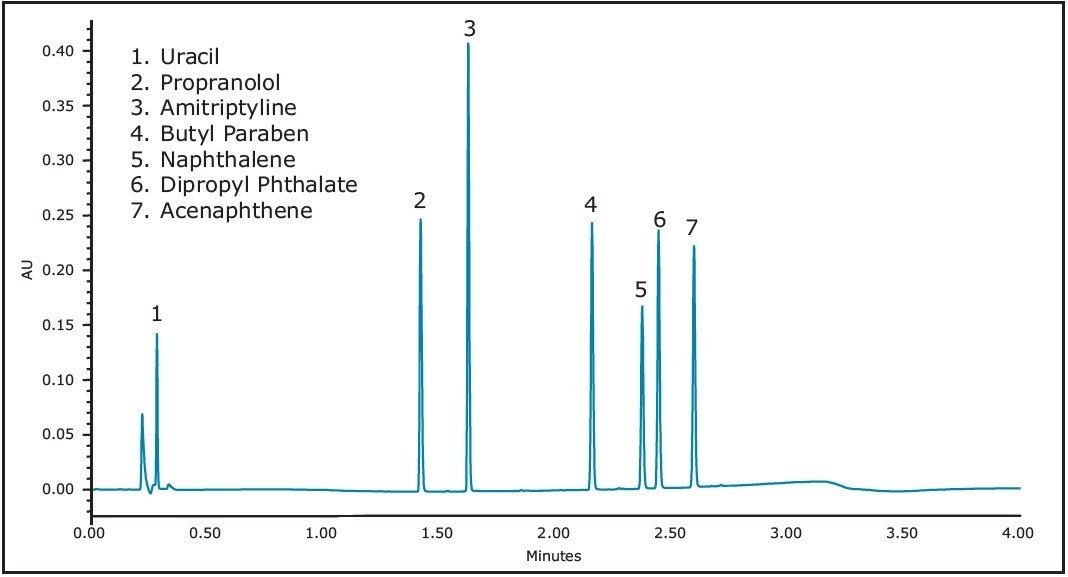
Uracil 0.016 mg/mL
Propranolol 0.400 mg/mL
Amitriptyline 0.100 mg/mL
Butyl Paraben 0.020 mg/mL
Naphthalene 0.060 mg/mL
Dipropyl Phthalate 0.340 mg/mL
Acenaphthene 0.200 mg/mL
Prior to testing, an ACQUITY UPLC and an ACQUITY UPLC H-Class were calibrated to ensure optimal system performance. Each system received a tray of 48 LCGC Certified Qsert Vials containing the QCRM (Figure 1). A total of eight vials per cap type per system were used. Eight individual injections per vial on each instrument were made, starting at vial time = 0 and ending at vial time = 56 h (8 h intervals).

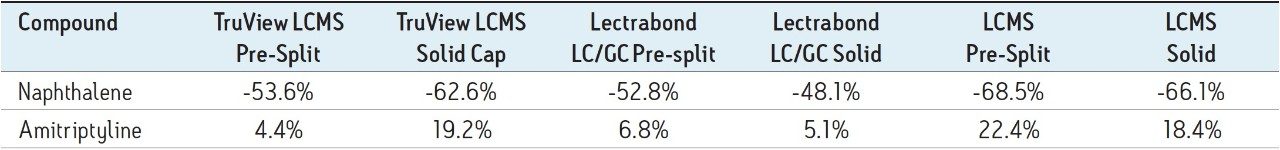
In the acquired chromatograms (e.g. Figure 2), the naphthalene peak was used to monitor changes in a volatile analyte, whereas the amitriptyline peak was used for to monitor changes in a non-volatile analyte. Changes in the peak areas for these two analytes (averaged across each set of 8 identical cap types) were calculated to obtain the total peak area % change for each analyte, injector type and cap type over the study time period. Trend plots of peak area % change vs. time were also created.
Using the fixed loop injector, the volatile compound naphthalene decreased in peak area during the study, with a range of -48% to -68% across the different cap types (Table 1). For the non-volatile compound amitriptyline, there was an increase in peak area, with a range of +4% to +22%.
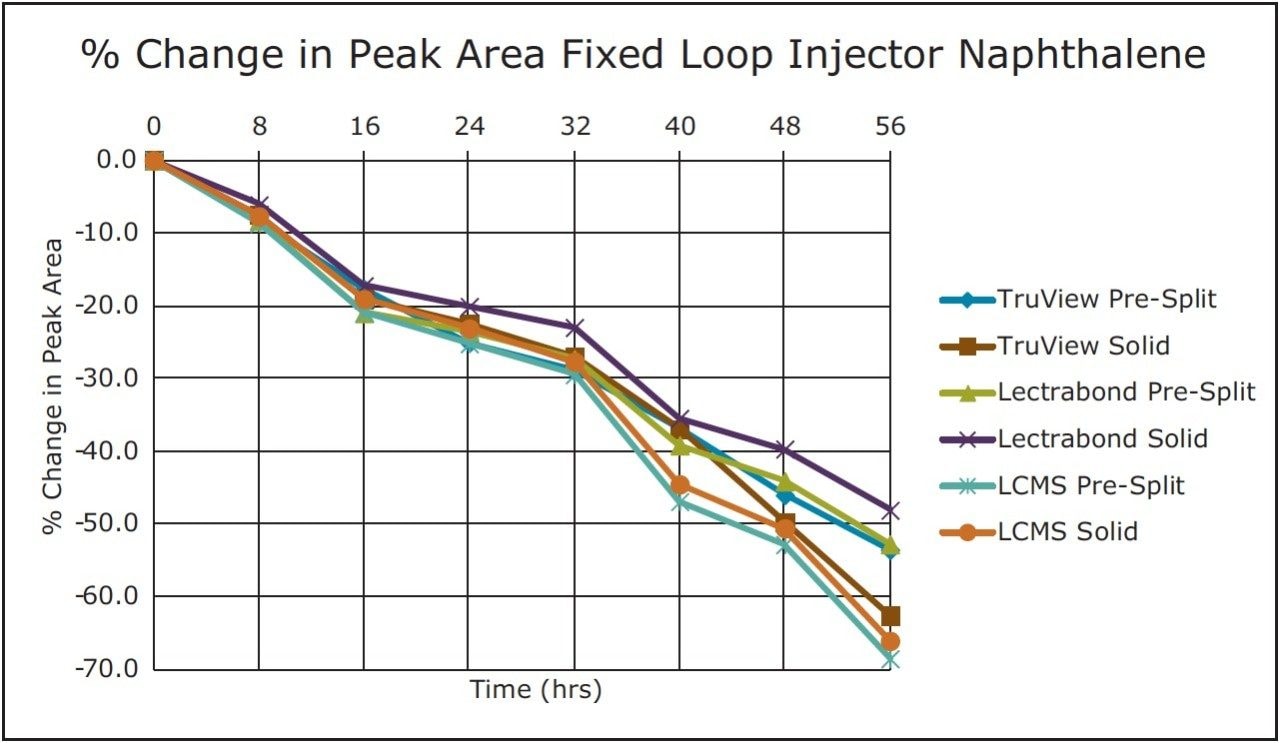
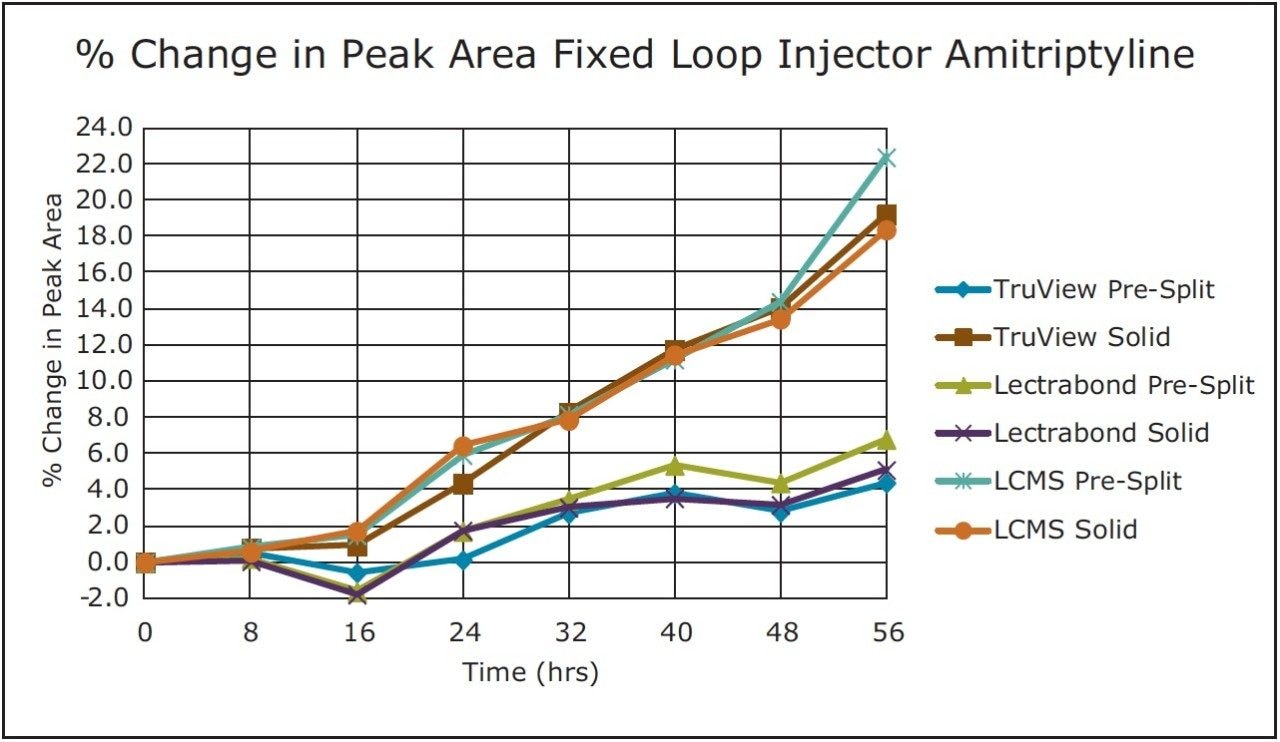
Trend plots of the peak areas for naphthalene and amitriptyline with these caps on the fixed loop injector are given in Figure 3 and 4, respectively.
When a vial cap septum is pierced and does not reseal sufficiently, evaporation can occur. If an analyte is volatile, it can evaporate and this can decrease the concentration/peak area of that analyte. If the diluent for the analytes is volatile (e.g. methanol), it can also evaporate and this can increase the concentration/peak area of all the analytes. The change in analyte concentrations and the resulting peak areas therefore depends on the two evaporation components: analyte vs. diluent volatility.
For the volatile compound naphthalene, the analyte evaporation component dominated. None of these caps prevented the significant change in concentration for this analyte over the study timeframe. For the non-volatile compound amitriptyline, there were smaller changes in this analyte concentration via diluent evaporation. Three vial cap types resealed sufficiently to keep that change under +7% over the study timeframe.
Using the flow through needle Injector (FTN), the volatile compound naphthalene decreased in peak area during the study, with a range of -26 % to -36% across the different cap types (Table 2). For the non-volatile compound Amitriptyline, four caps showed small peak areas increases and two caps showed small peak area decreases.
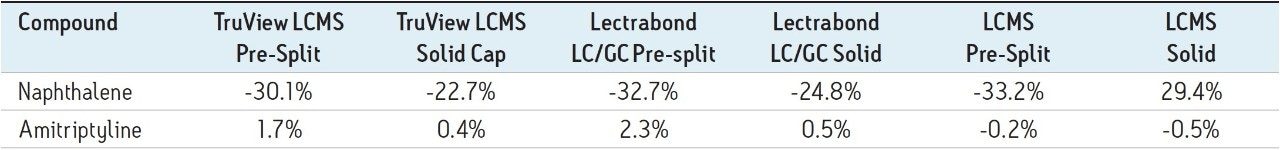
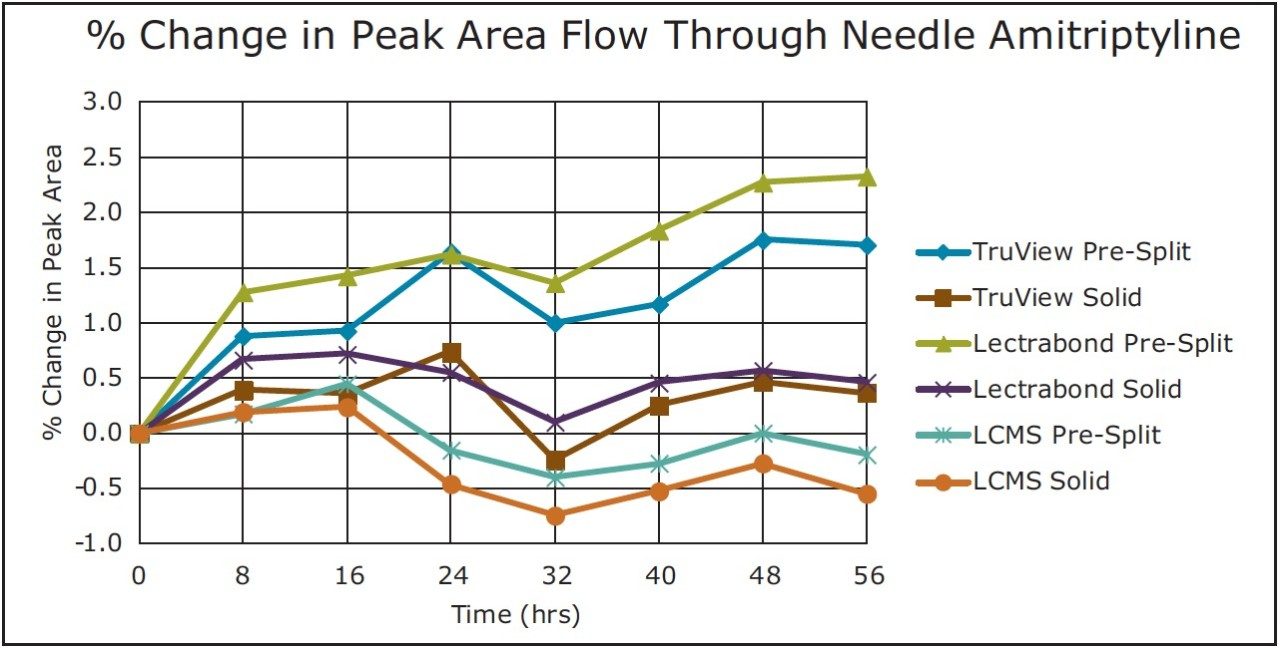
Trend plots of the peak areas for naphthalene and amitriptyline with these caps on the FTN are given in Figure 5 and 6, respectively.
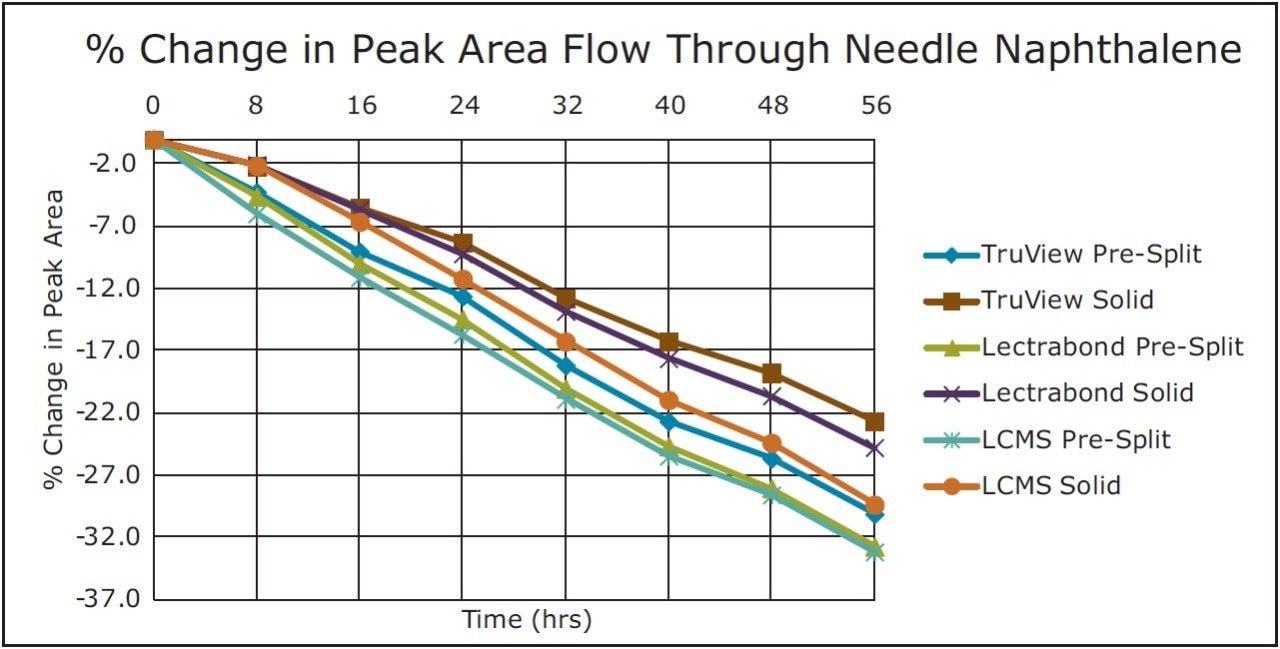
For the volatile compound naphthalene, the analyte evaporation component again dominated though the changes were about half that observed with the fixed loop injector. As above, none of these caps prevented the significant change in concentration for this analyte over the study timeframe.
For the non-volatile compound amitriptyline, there were only small changes in this analyte concentration via diluent evaporation. With the lower % peak area changes (TruView Solid, Lectrabond Solid, LCMS Pre-Split, LCMS Solid), there is some fluctuation in the data suggesting those changes may be within experimental sampling error. All the tested vial cap types resealed sufficiently to keep the % peak area change in the -1% to +3% range over the study timeframe.
The differences in results between the fixed loop injector and the flow through needle Injector (FTN) are due to the disparity in outside diameters of the septum piercing these injectors make (OD of 1.83 mm vs. 1.00 mm, resp.). The smaller flow through needle Injector (FTN) piercing allows for a better reseal after each injection. However, when good control over volatile analyte concentration is desired, the results suggest that frequent cap septa changes are advisable, regardless of the injector and cap type used.
For non-volatile analytes with a volatile diluent, the results depended on the injector used. With the fixed loop injector, the TruView Pre-Slit, Lectrabond pre-split and Lectrabond Solid septa gave better resealibility across the multiple injections and longer time periods. For the flow through needle Injector (FTN), there was much less difference among the different septa, with the TrueView Solid, Lectrabond Solid, LCMS Pre-Split and LCMS Solid septa performing best.
An important property of a vial cap is the ability to maintain the vial analyte concentration after multiple injections over time. This ability is influenced by different factors such as the volatility of the analyte/diluent, the number of injections, the elapsed time and the injector/cap septum used. The results of this study can provide guidance in this area. However, it is always best to change vial caps as soon as feasible after piercing. For critical analyses, analysts are advised to do analyte concentration/time course measurements on their systems to confirm a sufficient vial cap resealibility for their needs.
720005341, March 2015