Instrument Considerations for Successful Adaptation of Amino Acid Analysis Methods from an ACQUITY™ UPLC™ System to an ACQUITY Premier Binary Fixed Loop System
Abstract
Amino acid analysis is performed regularly for a wide range of samples including hydrolyzed proteins, cell culture, as well as food and feed samples. Waters™ created an ACQUITY UPLC AAA Solution which includes a pre-column derivatization kit, sub-2 µm columns and pre-packaged mobile phases suitable for UPLC analyses.2 With the advancement of new instrumentation, it is important to be able to migrate methods to newer systems. This study will demonstrate the considerations for adaptation of this pre-column derivatization method from the ACQUITY UPLC to the ACQUITY Premier Binary Fixed Loop System. After adaptation and optimization of the separation method, performance will be demonstrated, including linearity, repeatability, reproducibility, limit of quantitation, and limit of detection.
Benefits
- ACQUITY Premier Binary System provides exceptional precision for challenging gradients and increased speed for high throughput analysis
- All critical performance characteristics are maintained, including peak shape, resolution, linearity, limit of quantification, and intermediate precision after method adaptation
- AccQ•Tag™ Ultra Chemistry Kit, which includes column, standards, eluents, and derivatization kit allows for fast, reliable and reproducible amino acid derivatization, separation, and quantification
- The ACQUITY Premier Binary System features MaxPeak High Performance Surfaces (HPS) Technology, which increases analyte recovery, sensitivity, and reproducibility by minimizing analyte/surface interactions that can lead to sample losses
Introduction
Analysis of amino acids (AAA) can be very challenging due to low or complete lack of UV absorbance and the wide range of chemical properties of amino acids. Given these challenges, pre-column derivatization using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) followed by a reversed-phase separation has been found to be a highly reproducible technique for quantitative analysis of amino acids using a UHPLC/UPLC System.1 However, new systems are constantly being introduced and migration of the amino acid solution while maintaining method performance is crucial. In this work, a legacy method developed on the ACQUITY UPLC will be migrated to a newer LC platform, the ACQUITY Premier Binary Fixed Loop System, which includes some design differences that may impact the method migration. Some method modifications were required to account for these differences and the final method conditions yielded nearly identical results when compared to the original method run on the ACQUITY UPLC System.
Experimental
All calibration standards were prepared from Waters Amino Acid Standard (p/n: WAT088122) using norvaline (p/n: 186009301) as the internal standard and 0.1 N HCl as the diluent. The internal standard stock was prepared at 2500 µM in 0.1 N HCl. The final concentration of the calibrants were 1, 5, 10, 20, 50, 100, 200, and 500 µM for all amino acids (except cysteine which was present in ½ the concentration) and 250 µM for norvaline (internal standard).3 The precision sample was prepared at 500 µM (250 µM cysteine). Norvaline was kept constant at 250 µM.
LC Conditions
|
LC systems: |
ACQUITY UPLC System with passive preheating and in-line filter ACQUITY Premier Binary Fixed Loop System with active column preheating and in-line filter |
|
Detection: |
ACQUITY UPLC System– ACQUITY TUV Detector with 10 mm UPLC Analytical Flow Cell and CH-A Column Heater ACQUITY Premier Binary Fixed Loop System – ACQUITY TUV Detector with 10 mm UPLC Analytical Flow Cell and CH-A Column Heater |
|
Wavelength: |
260 nm |
|
Sampling rate: |
10 Hz |
|
Vials: |
LCGC certified clear glass 12 x 32 mm screw neck vial, total recovery with cap and PTFE/Silicone septum (not pre-slit) (p/n: 186000384C) |
|
Column(s): |
AccQ•Tag Ultra C18, 1.7 µm 2.1 x 100 mm (p/n: 186003837) |
|
Column temperature: |
55 °C (for hydrolysate & food and feed), 60 °C (for cell culture) on the ACQUITY UPLC System 55 °C (for hydrolysate & food and feed), 50 °C (for cell culture) on the ACQUITY Premier Fixed Loop System |
|
Sample temperature: |
20 °C |
|
Injection volume: |
1 µL (ACQUITY UPLC System) and 0.5 µL (ACQUITY Premier Binary Fixed Loop System) |
|
Injection mode |
Partial Loop Needle Overflow (PLNO using default settings) |
|
Needle Size: |
2 µL HPS, Ext Hydro Tip (p/n: 700013814) |
|
Flow rate: |
0.7 mL/min |
|
Mobile phase A: |
1:20 dilution of AccQ•Tag Ultra Eluent A (p/n: 186003838) for hydrolysate & food and feed 1:10 dilution of AccQ•Tag Ultra Eluent A for cell culture |
|
Mobile phase B: |
AccQ•Tag Ultra Eluent B (p/n: 186003839) |
|
Weak needle wash: |
95:5 (v/v) Water:Acetonitrile (ACQUITY UPLC and ACQUITY Premier Binary Fixed Loop Systems) |
|
Strong needle wash: |
5:95 (v/v) Water:Acetonitrile (ACQUITY UPLC System) 95:5 (v/v) Water:Acetonitrile (ACQUITY Premier Binary Fixed Loop System) |
|
Seal wash: |
50:50 (v/v) Water:Acetonitrile (ACQUITY UPLC and ACQUITY Premier Binary Fixed Loop Systems) |
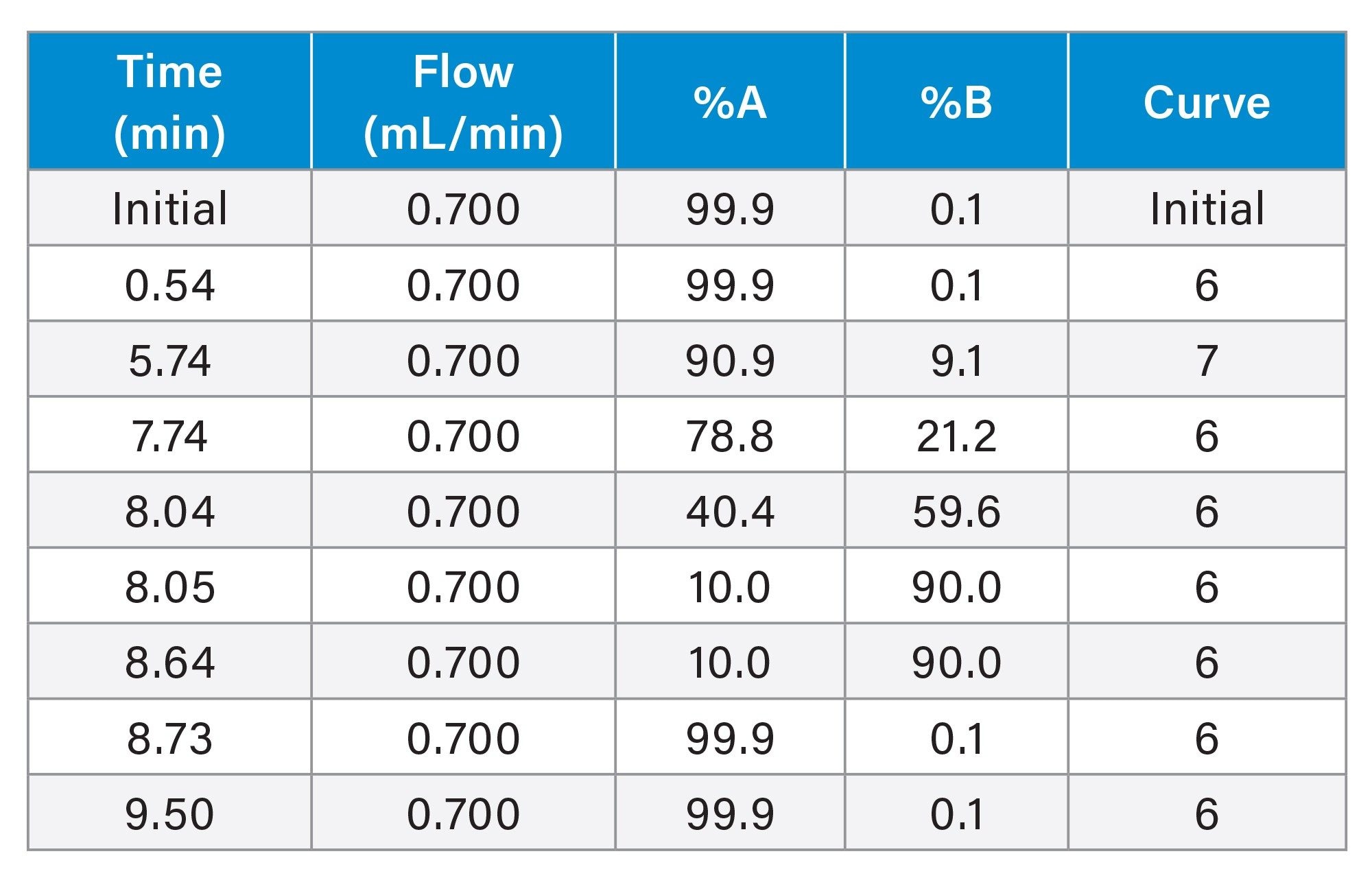
Gradient Table
Data Management
|
Chromatography data system: |
Empower™ 3, FR 3.7.0 |
Results and Discussion
The separation of a wide range of amino acids is challenging as slight changes in temperature, gradient delivery, and other conditions can impact retentivity and selectivity. Given these challenges, differences across LC Systems can require optimization of the method on a system-by-system basis. For these studies, the method conditions for the separation of amino acids from protein hydrolysate were adapted from the separation on a 2.1 x 100 mm from the amino acid total solution on the ACQUITY UPLC System.1 Pre-column derivatization of amino acids was performed using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC), followed by separation of the derivatives with reversed-phase liquid chromatography.1
Instrument Considerations
The instrument method from the ACQUITY UPLC AccQ•Tag Ultra Solution was tested on the ACQUITY Premier Binary Fixed Loop System. Adjustment of the method was required to achieve adequate resolution and chromatographic performance. To assess the final method conditions, both the ACQUITY UPLC and ACQUITY Premier Binary Fixed Loop Systems were tested. Both systems consist of binary pumps, fixed loop sample managers, and TUV (tunable wavelength) detectors. Differences included passive preheating on the ACQUITY UPLC System and active column preheating on the ACQUITY Premier Binary Fixed Loop System, along with slight differences in system dispersion.
Method Optimization of HPLC Amino Acid Separation
To ensure no differences could be attributed to the standards and samples, the standards and samples were prepared, derivatized, pooled, and split between the two systems. Mobile phases were also made up in large batches and split between the two systems. The same column was used between the two systems to reduce any column variability.
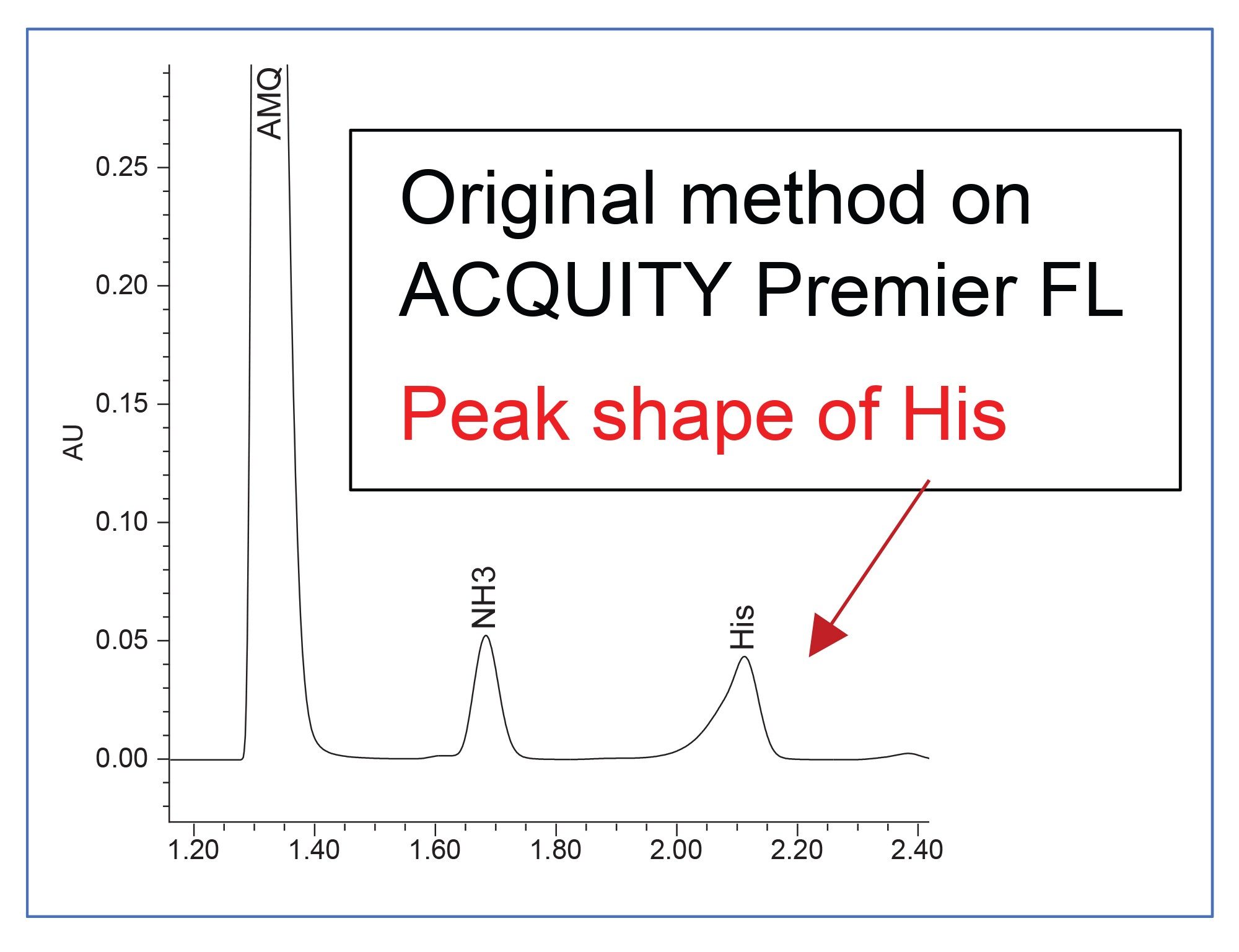
Direct migration of the method with no adjustments resulted in peak distortion of histidine on the ACQUITY Premier Binary Fixed Loop System, likely due to strong solvent effects caused by lower system dispersion (Figure 1). The ACQUITY Premier Binary Systems offer the needed sensitivity for amino acid analysis, precision for challenging gradients, and increased speed for high-throughput analysis.2 Several method adjustments were evaluated including changing the strong needle wash from 5:95 water:acetonitrile to 95:5 water:acetonitrile.
Finally, adjustment of the injection volume was examined. After derivatization, the injection solvent contains a relatively high amount of organic (20%) when compared to the method starting conditions, therefore decreasing the injection volume could improve histidine peak shape. The ACQUITY Premier Binary Fixed Loop System produced significantly greater peak distortion for histidine above 0.5 µL, thus for this system a 0.5 µl injection volume was determined to be acceptable (Figure 2). Sensitivity was also evaluated due to the decrease of the injection volume by 50%.
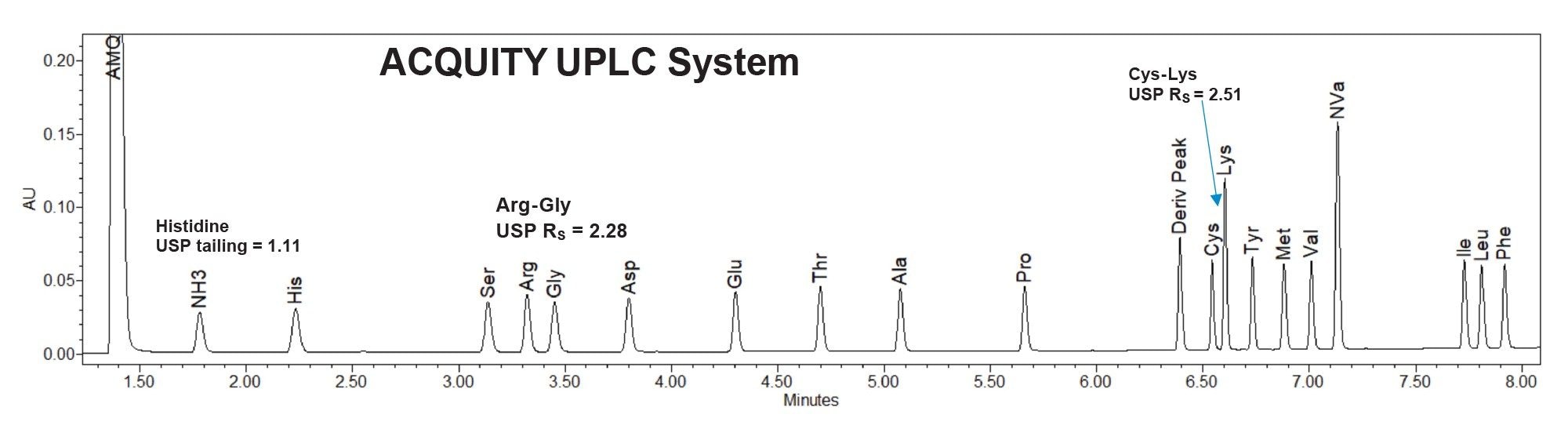
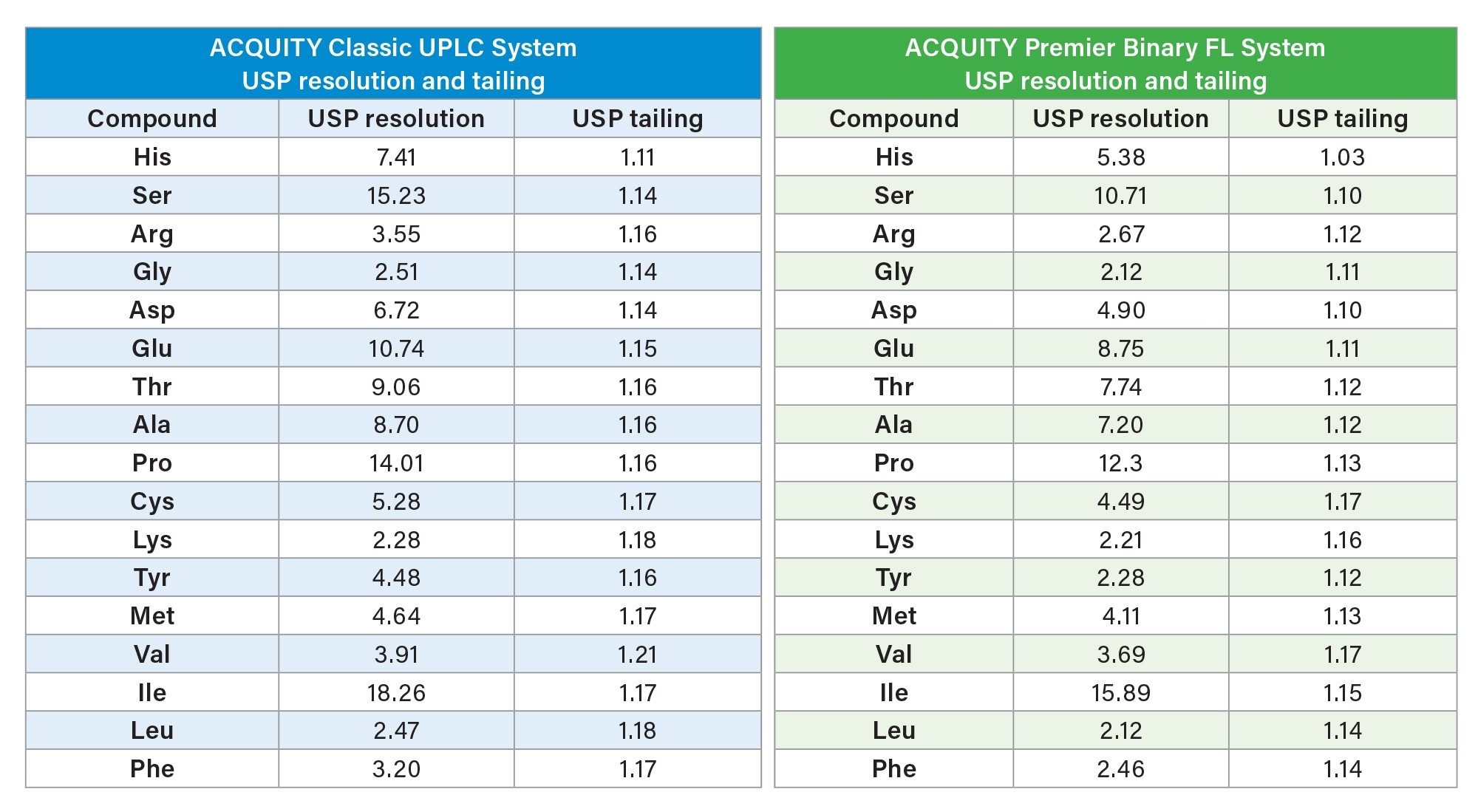
After adjustment of the injection volumes for each system, chromatographic performance was compared using the hydrolysate standard (Table 1). Both systems were found to have acceptable USP resolution (>2.0) and peak shape for the hydrolysate amino acids (Figures 2 and 3).
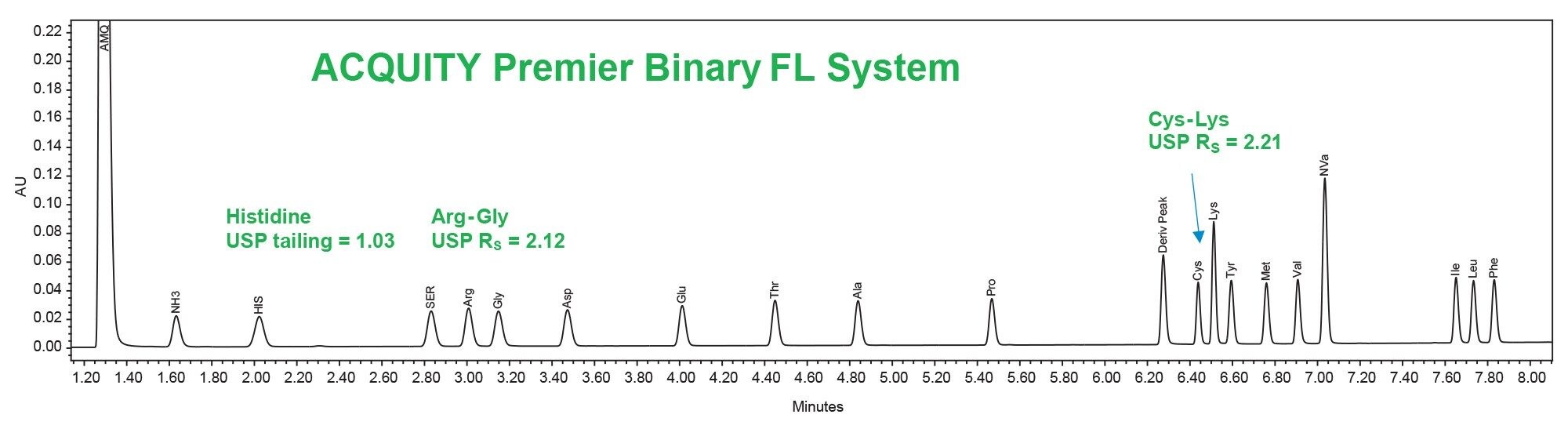
Verification of Amino Acid Analysis on ACQUITY UPLC and ACQUITY Premier Binary Fixed Loop Systems
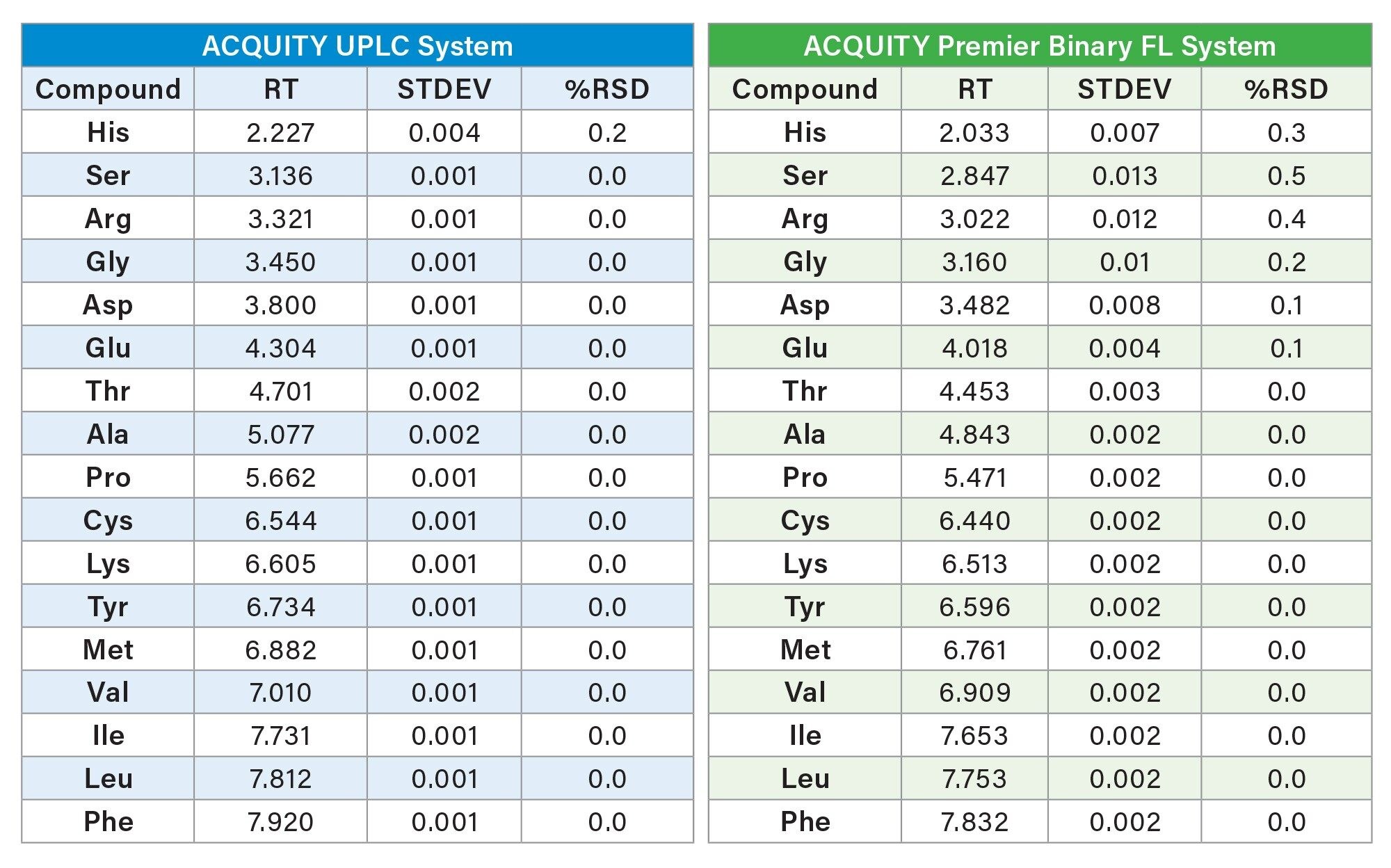
Using the adjusted injection volume (0.5 uL), each system was evaluated using the protein AA hydrolysate standard to ensure adequate repeatability for both retention time and peak area. The results were comparable across both binary systems. All retention %RSDs were <0.5% for the 500 pmol standard (25 pmol on column) for six replicate injections.
The area precision for each system also produced comparable results. The ACQUITY UPLC System produced area RSDs from 0.2% to 0.4% for all the hydrolysate amino acids. For the ACQUITY Premier Binary Fixed Loop System produced area %RSDs were 1.1% to 1.4% for the hydrolysates. Although slightly lower area counts and higher %RSDs were observed on the ACQUITY Premier Binary Fixed Loop System, both systems demonstrated good area repeatability. Results for both retention time and peak area reproducibility are shown in tables 2 and 3.
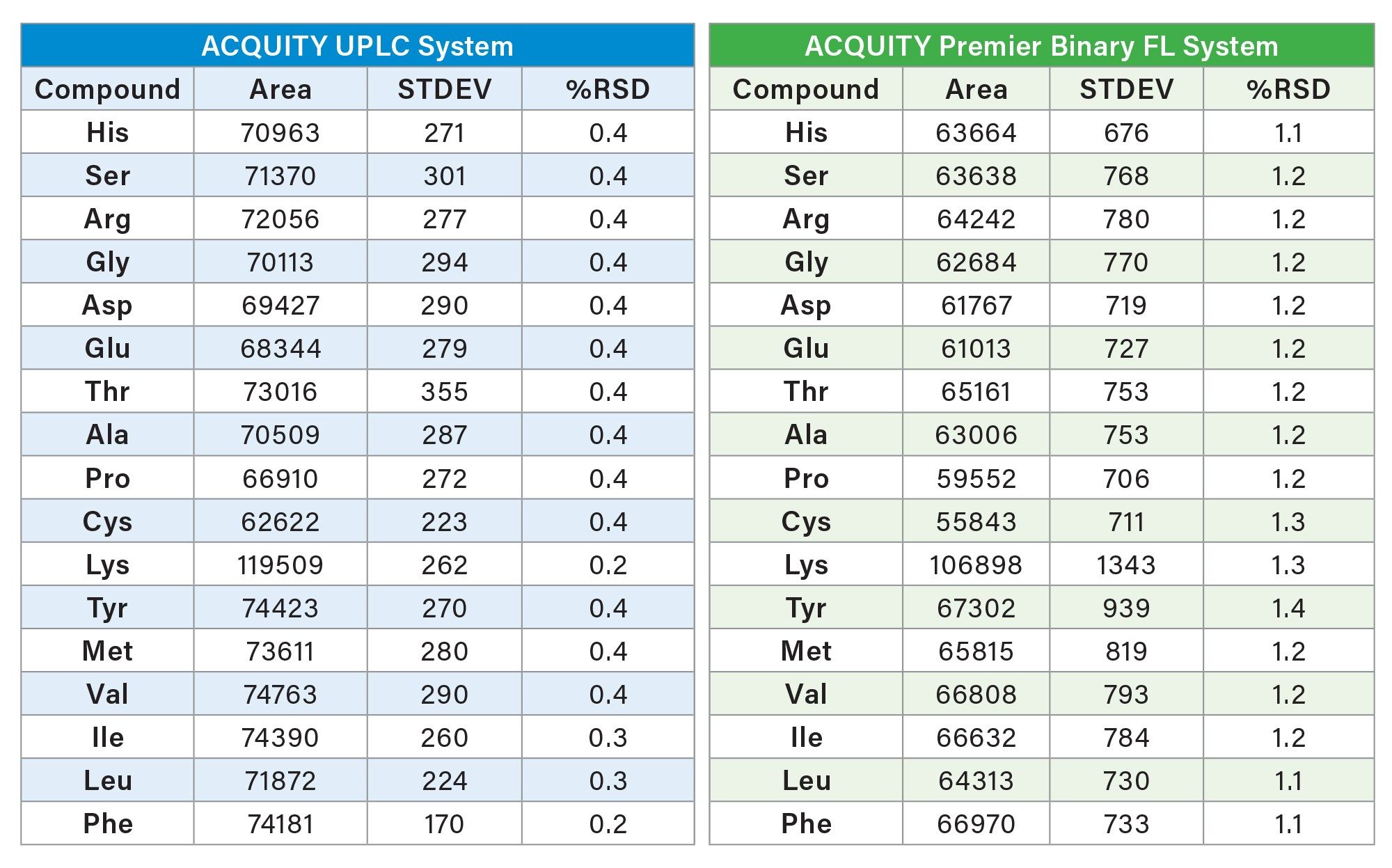
Intermediate Precision for Retention Time and Area RSD
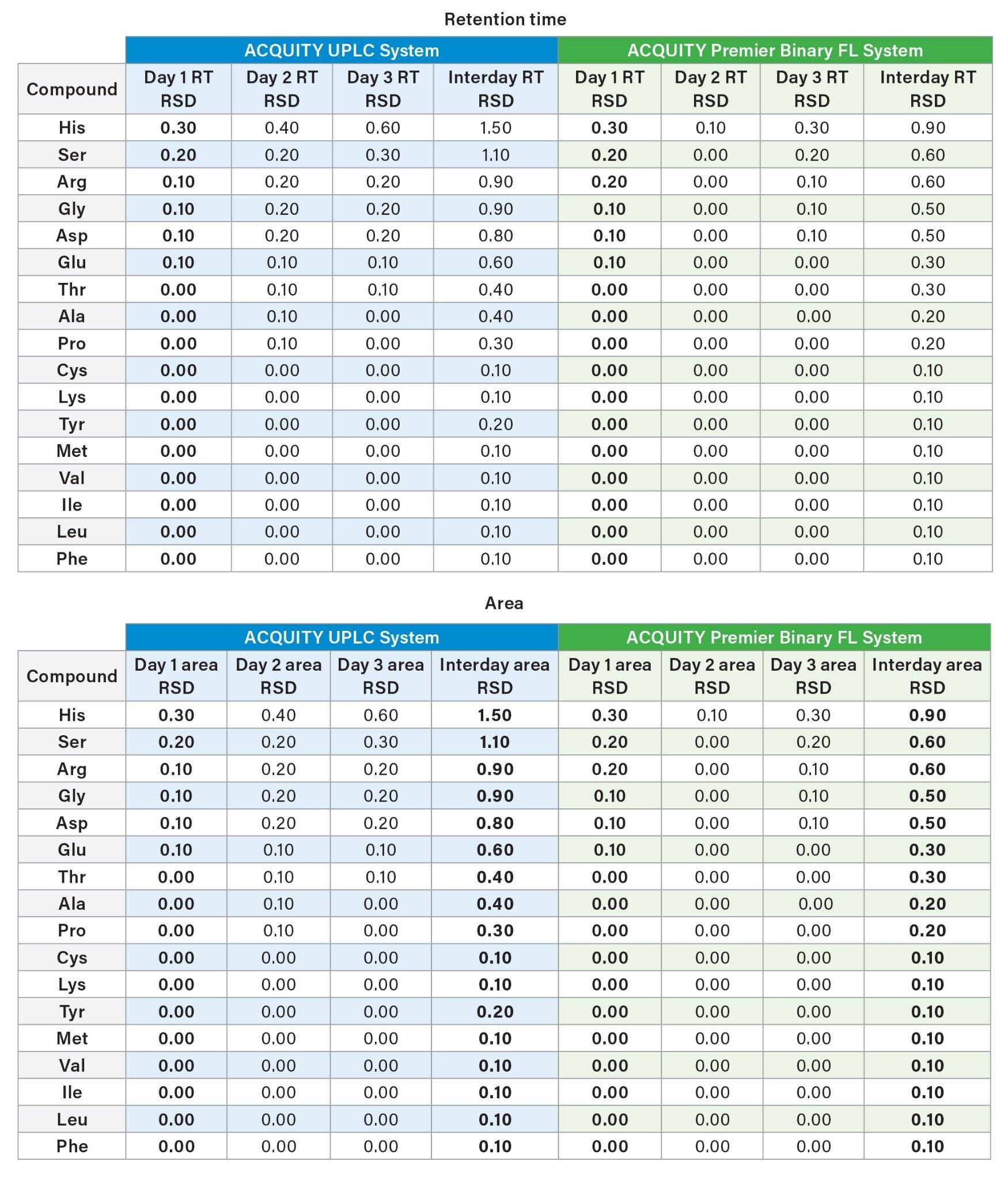
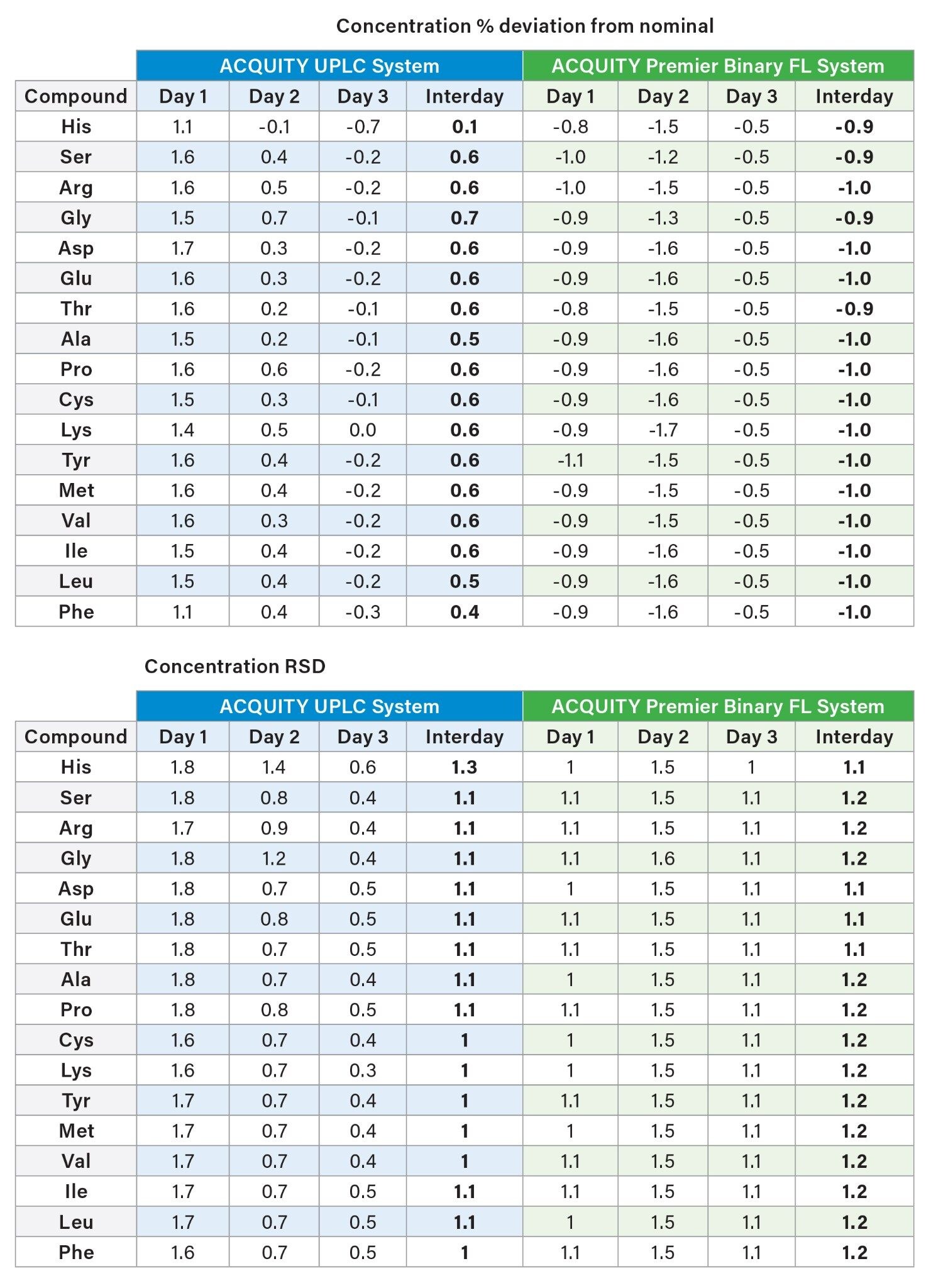
In addition to repeatability, intermediate precision is essential for analysis and quantitation of amino acids. Both the ACQUITY UPLC and ACQUITY Premier Binary Fixed Loop Systems were tested over three days with six samples tested per day. An internal standard (norvaline) was used to normalize differences in sample preparation. The analysis evaluated the intra-day precision for six injections and the aggregate precision across all three days for a total of 18 injections.
The results on both systems demonstrated high intra-day retention time precision and area precision. All retention times were within 1.5% RSD over the course of three days, with a precision that is well within the expected retention time range for each system. The peak area %RSD were within 1.8% within one day and 2.2% across all three days. The results demonstrate the reproducibility of the analysis on both the ACQUITY UPLC and ACQUITY Premier Binary Fixed Loop Systems.
Linearity and Limit of Detection/Limit of Quantitation
To ensure accurate quantitation of all the amino acids, linearity as well as limit of detection (LOD) and limit of quantitation (LOQ) were evaluated for both the ACQUITY UPLC System and the ACQUITY Premier Binary Fixed Loop System. Linearity was determined by injecting eight concentration levels of hydrolysate standard ranging from 1 µM to 500 µM. The calibration curves for both instruments resulted in an acceptable R2 of >0.99 for all amino acids. Given the distribution of standards across the dynamic range, a weighting of 1/x was used for the calibration curve. The limit of quantitation (LOQ), as defined by signal-to-noise equal to or greater than 10:1, was 1 µM for both systems. All values above the LOQ exhibited deviations of <17% and deviations of <30% at the LOQ. Despite the differences in injection volume, the limit of detection (LOD) was determined to be 0.5 µM based on a signal to noise ratio of 3:1 for both instruments.
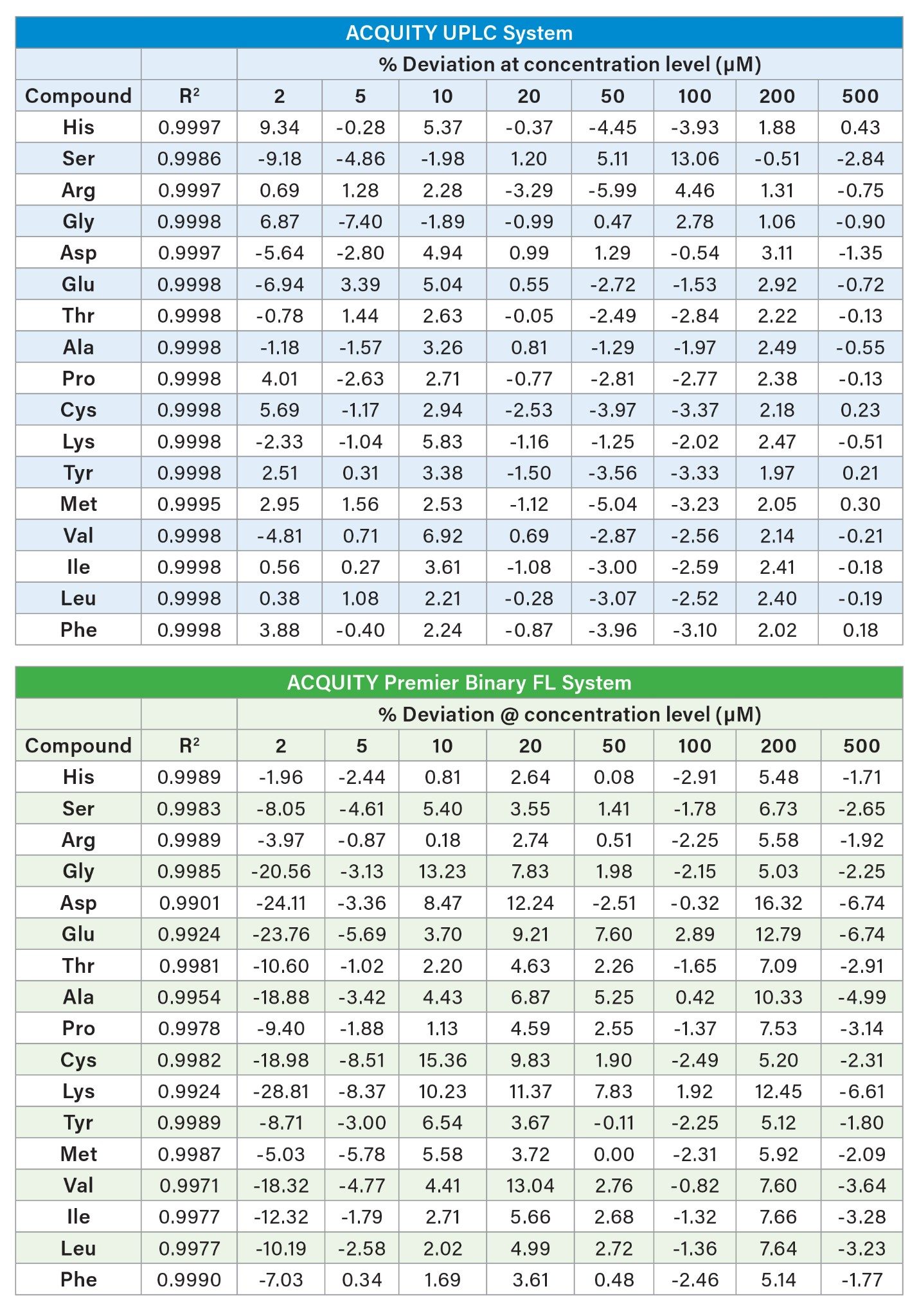
Multi-day concentration % deviation from nominal and concentration RSDs were calculated. Low % deviation and low RSDs were observed across the three days for both systems.
Separation of Additional Amino Acid Standards
Separation of additional amino acid standards was performed on the ACQUITY Premier Binary Fixed Loop System with a 0.5 µL injection volume. To achieve adequate separation, the cell culture standard required a column temperature of 50 °C. Food and feed standard separation was at 55 °C.
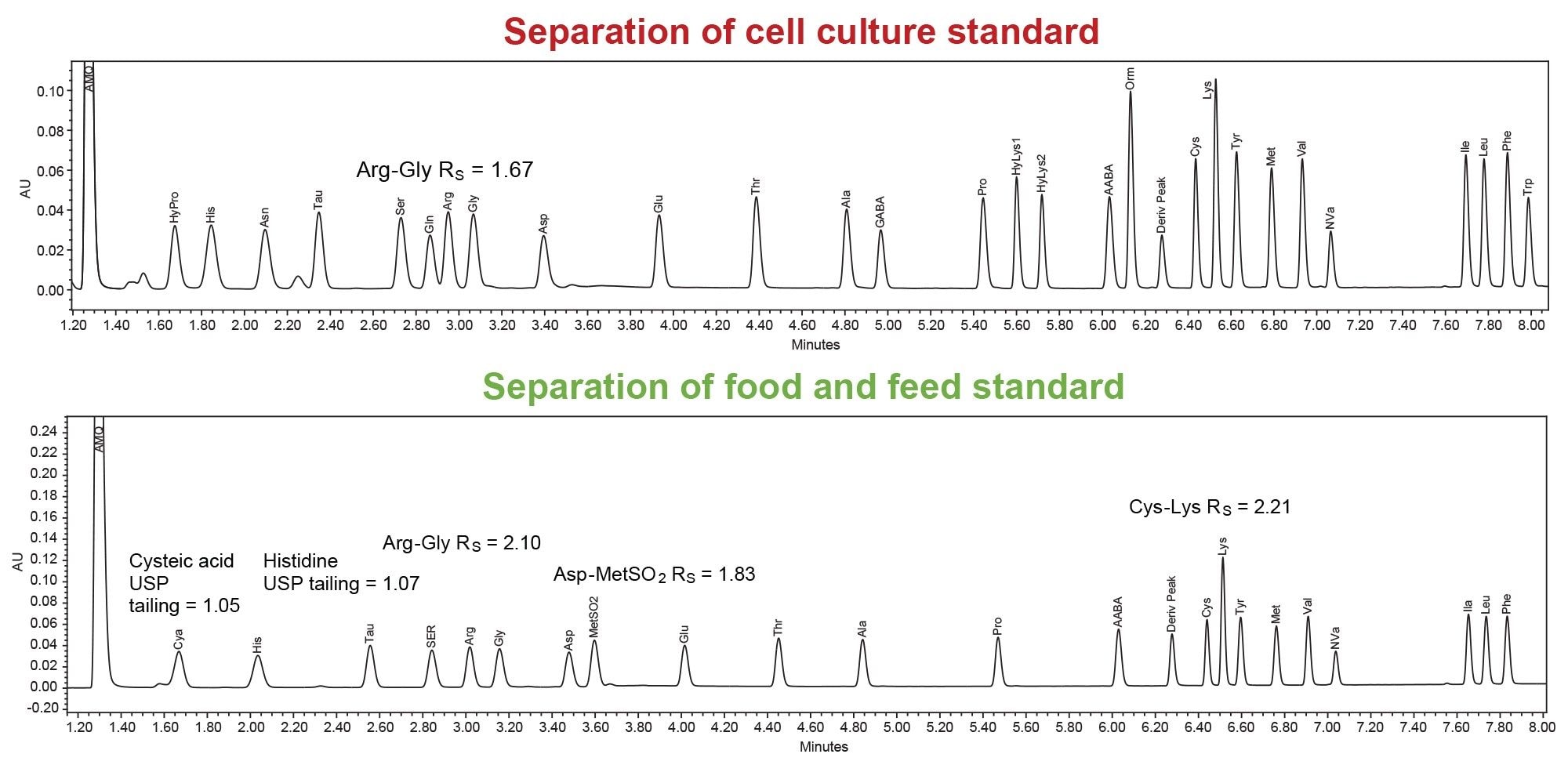
As many customers need to analyze both hydroxyproline and cysteic acid in samples, the food and feed standard was spiked with hydroxyproline. Both amino acids elute early in the gradient, at <3% organic, and may require a binary pump for reproducible separation. Successful separation was achieved and is shown in Figure 5.

Quantitative Analysis of Energy Drink
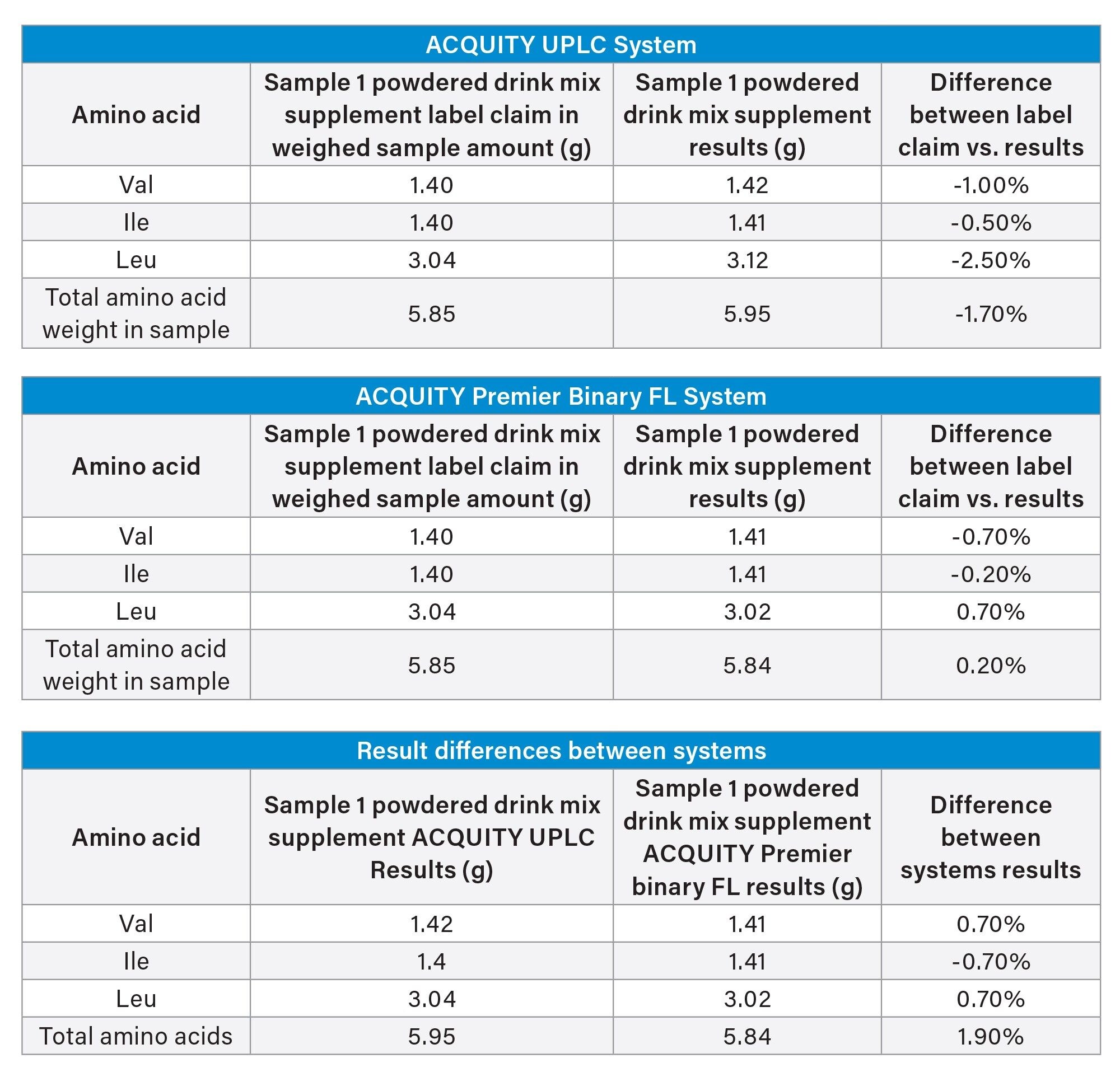
Quantitative analysis of an amino acid powdered drink mix was performed on the ACQUITY UPLC and ACQUITY Premier Binary Fixed Loop Systems. The method provided comparable results for the amino acid content in the powdered drink mix sample. The chromatograms for the standard and the sample are shown in Figure 6 for the ACQUITY Premier Binary Fixed loop System. The low % difference between the two systems indicates high quantitative reproducibility (Table 7).
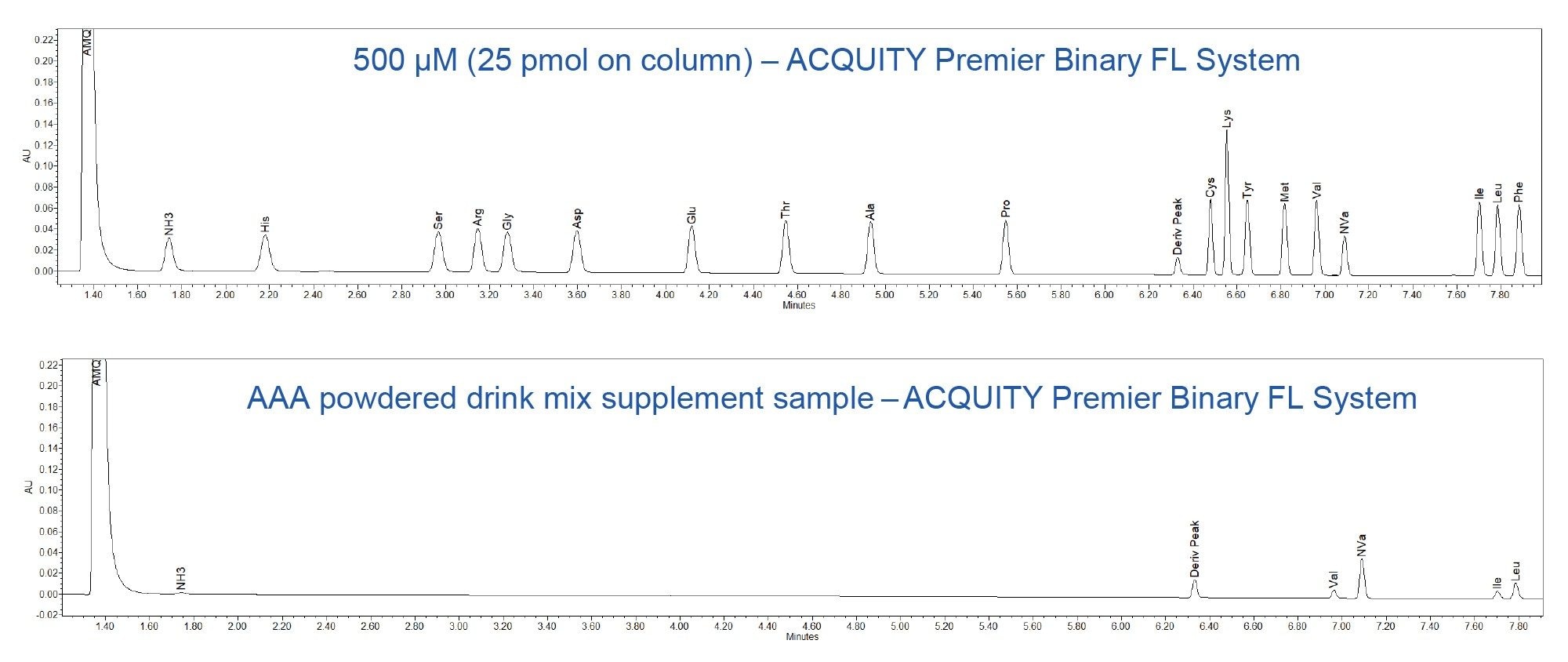
Conclusion
The AccQ•Tag Ultra method on the ACQUITY UPLC System was successfully migrated to the ACQUITY Premier Binary Fixed Loop System. The injection volume was changed from 1 µL in the original method to 0.5 µL to reduce the impact of strong solvent effects on histidine peak shape. Method performance was evaluated on both systems to ensure the results for linearity, precision, intermediate precision, LOD, and LOQ were acceptable after the adjustment of injection volume. Both systems showed similar overall performance, which was demonstrated by low % RSDs and precise quantitative results.
Additionally, standards for cell culture and foods and feeds were also analyzed. To facilitate adequate peak shape and resolution, the cell culture method required adjustment of the column temperature from 60 °C to 50 °C. The food and feed standard spiked with hydroxyproline was successfully separated using only the decreased injection volume.
Finally, two powdered drink mix samples were analyzed using the adjusted method to compare quantitative results acquired using both systems. The results for the quantitation of the amino acid content in the powdered drink mix sample showed good agreement between the two systems and the label claims for the sample. This is indicative of high quantitative reproducibility across the two systems.
References
- Amino Acid Analysis Application Notebook, 720006130, 2020.
- Jennifer Simeone, Paula Hong. Instrument Considerations for Successful Adaptation of Amino Acids Analysis Methods Which Utilize Pre-Column Derivatization from an ACQUITY UPLC to an ACQUITY Premier Binary System. Waters Application Note, 720007440, 2021.
- Amino Acid Standard Kits Care and Use Manual. Waters Care and Use Manual, 72000663.
720008073, October 2023