Deploying Auto•Blend Plus Technology on the ACQUITY™ Premier UPLC™ System
Abstract
This study demonstrates the value and utility Auto•Blend Plus technology can offer in the development and manufacturing of biopharmaceutical drug products. As a standard capability offered with the ACQUITY™ Premier UPLC System, Auto•Blend Plus technology reduces the burden and costs associated with the method development process for charge variant analysis. This is accomplished by taking advantage of the quaternary blending capabilities of the ACQUITY Premier QSM module to manage the mobile phase formulation using reservoirs of pure solvents or concentrated stock solutions. This allows users to reduce time at the bench and spend more time analyzing data. To demonstrate this, a nondenaturing ion-exchange chromatography (IEX) method was developed for a mAb-based drug product using a single set of mobile phase components to scout across multiple conditions. Results indicate the ACQUITY Premier System in combination with the Auto•Blend Plus technology offers an efficient solution in method scouting and optimization. Furthermore, user accessible composition tables allowed for full transparency of the optimized method, streamlining both method validation and eventual migration to regulated environments using a defined set of mobile phase components. These features, which are fully integrated across Waters CDS/informatics solutions, makes the ACQUITY Premier System an ideal platform to support the development and manufacturing of biopharmaceutical drug products.
Benefits
- Auto•Blend Plus technology offers:
- Flexible solvent management for efficient method scouting
- Ability to reduce costs and time utilizing a single simple set of mobile phases
- Full integration with Waters compliant-ready Empower™ Software
- Ability to validate and migrate methods using integrated composition tables
Introduction
The method development process associated with pharmaceuticals can at times be an arduous task comprised of evaluating multiple separation method parameters in an iterative fashion. This optimization process is not only resource heavy in terms of bench time spent in the lab, but also costly, as each condition set investigated typically requires a new sample and mobile phase preparation. This is particularly true for the biopharmaceutical industry where protein-based samples are often assayed using non-denaturing techniques such as IEX, which require optimization of conditions related to pH and ionic strength to adequately resolve charge variants related to drug products.1,2 In this respect, deploying technology which can reduce the burden associated with method development cycles would be beneficial to pharmaceutical companies and consequently help drive products to market faster.
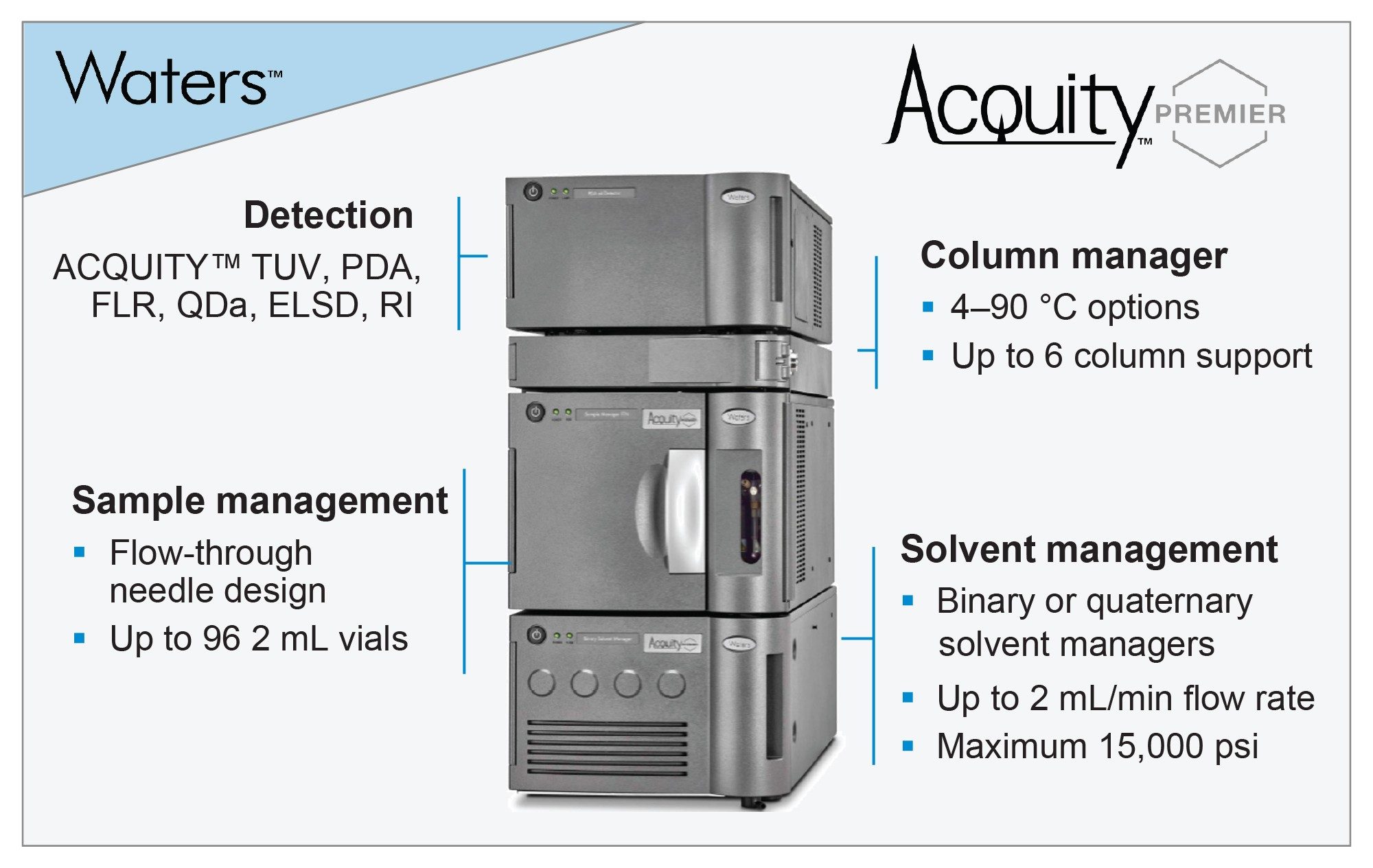
Recently, Waters introduced the ACQUITY Premier UPLC System featuring MaxPeak™ HPS technology (Figure 1) which further improves upon the chromatographic performance established by Waters LC™ Bioseparations portfolio. As part of this offering, the ACQUITY Premier System can be configured with a binary or quaternary solvent manager. The latter allows customers to take advantage of Waters Auto•Blend Plus technology to streamline the method development process. This is achieved through the utilization of the quaternary blending capabilities to manage real time mobile phase formulation using reservoirs of concentrated stock solutions, enabling users to reduce time at the bench and more time analyzing data. The objective of this study is to apply Auto•Blend Plus technology for the optimization of a charge variant separation of Infliximab, and demonstrate its utility for speeding up the drug development process and reducing development costs. 3,4
Experimental
Sodium phosphate (monobasic/dibasic) was purchased from Sigma Aldrich, Sodium chloride was purchased from Fisher Chemical. Stock solutions were prepared as 100 mM NaH2PO4, 100 mM Na2HPO4, and 1000 mM NaCl using MS-grade water. The Infliximab drug product Remicade MaxPeak™ HPS technology was purchased from Amerisource Bergen and prepared at the dosage concentration (10 mg/mL) as per manufacturer’s instructions and injected neat.
LC Conditions (Auto•Blend Plus technology)
|
LC system: |
ACQUITY Premier System (QSM-variant) |
|
Detection: |
ACQUITY TUV, FC=Ti 5 mm, λ=214 nm |
|
Vials: |
QuanRecovery MaxPeak Vials, (p/n: 186009186) |
|
Column(s): |
Protein-Pak™ Hi Res CM (7 µm, 4.6 × 100 mm, p/n: 186004929) |
|
Column temperature: |
40 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
5 µL |
|
Flow rate: |
0.750 mL/min |
|
Mobile phase A: |
100 mM NaH2PO4 |
|
Mobile phase B: |
100 mM Na2HPO4 |
|
Mobile phase C: |
1000 mM NaCl |
|
Mobile phase D: |
H2O |
LC Conditions (Conventional Method)
|
LC system: |
ACQUITY Premier System (QSM-variant) |
|
Detection: |
ACQUITY TUV, FC=Ti 5mm, λ=214 nm |
|
Vials: |
QuanRecovery MaxPeak™ Vials |
|
Column(s): |
Protein-Pak HiRes CM (7 µm, 4.6×100 mm) |
|
Column temperature: |
40 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
5 µL |
|
Flow rate: |
0.750 mL/min |
|
Mobile phase A: |
20 mM phosphate buffer, pH 6.55 |
|
Mobile phase B: |
20 mM phosphate buffer with 200 mM NaCl, pH 6.45 |
|
Mobile phase C: |
H2O |
|
Mobile phase D: |
H2O |
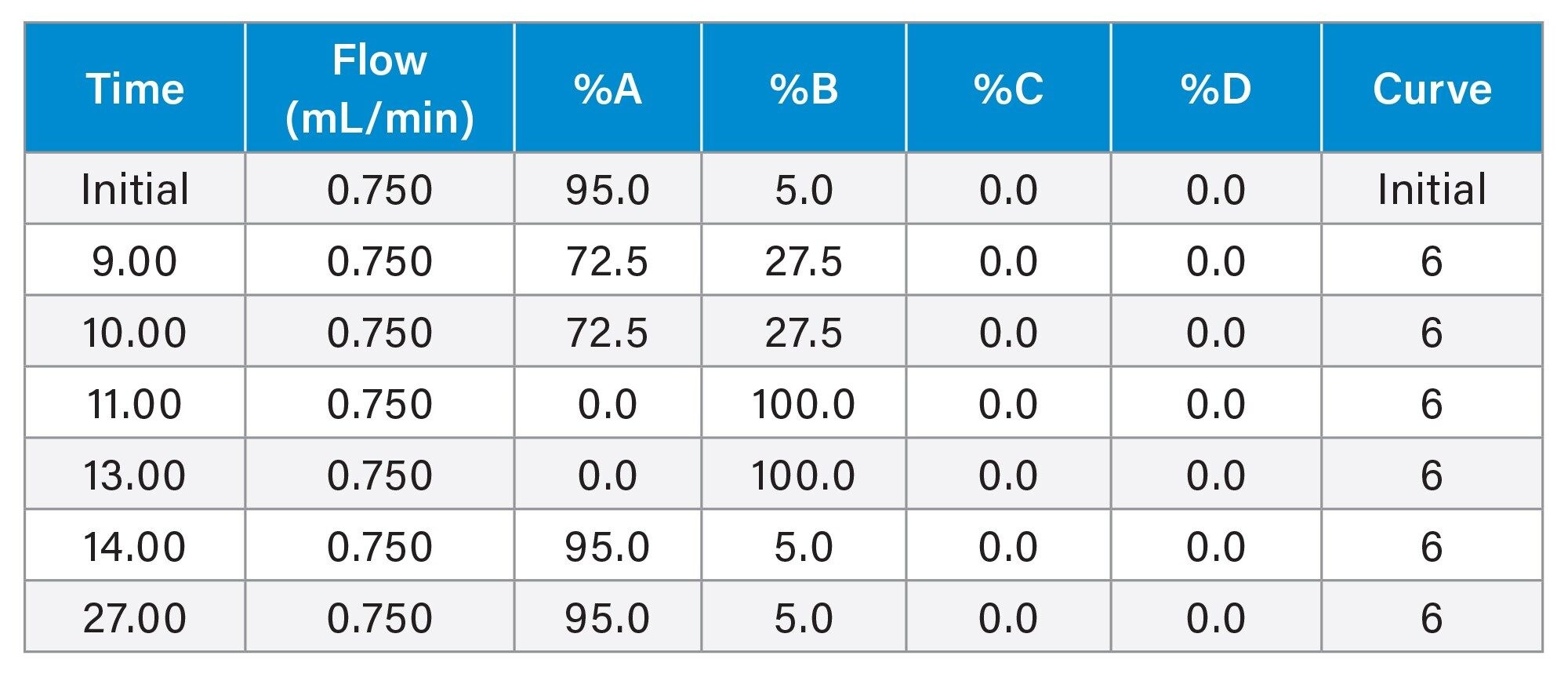
Gradient Table (Conventional Method)
Data Management
|
Chromatography software: |
Empower 3, FR4 |
Results and Discussion
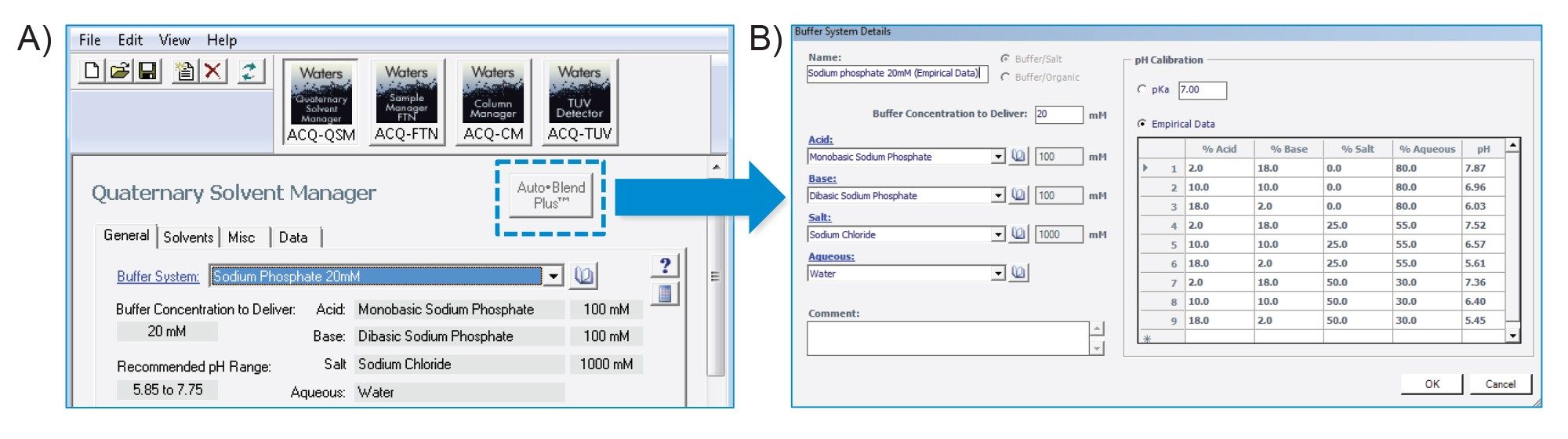
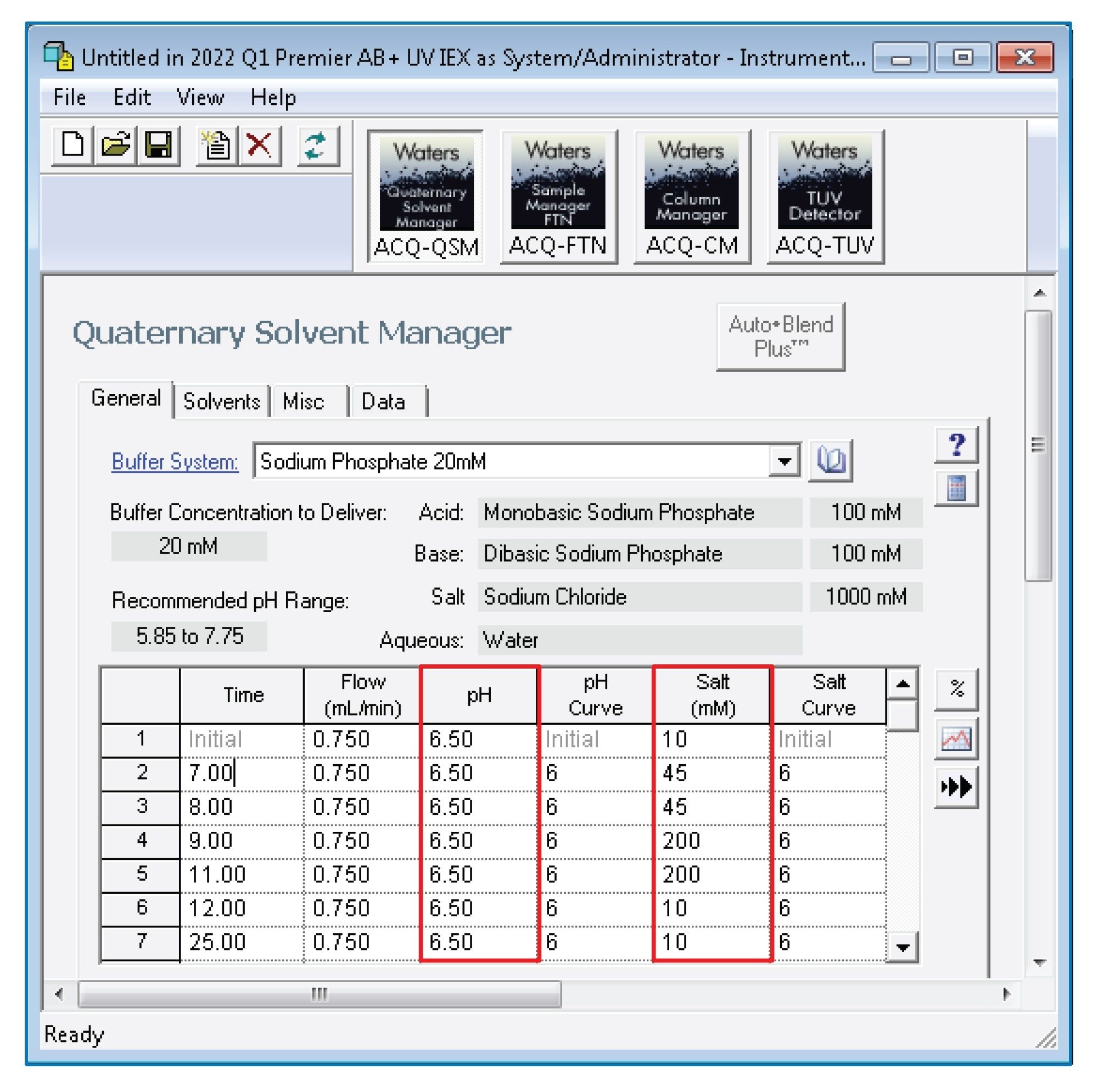
As part of the standard capabilities of the ACQUITY Premier System, Auto•Blend Plus methods can be deployed via the instrument method panel of recent Empower, MassLynx™, and waters_connect™ Software releases. In this study, as shown in Figure 2A, Empower 3 was used to enable Auto•Blend Plus technology by selecting the associated icon (dash-lined box) in the instrument method window. Once selected, the Auto•Blend Plus interface will open as shown in Figure 2B. From this interface a user can choose from pre-populated pull-down lists to select between aqueous buffers or organic solvents or use custom defined reservoir systems. As shown in the figure, when using a custom reservoir system, users have the ability to calibrate pH using either pKa or empirical data. The empirical data method is of particular interest as it allows the Auto•Blend Plus algorithm to correct for the common-ion effect introduced by the salt in solution, improving its ability to maintain an accurate pH across a range of ionic strength. To take advantage of the empirical data method we experimentally measured pH at nine different mobile phase compositions using the stock solution shown in the instrument method table. An example of this is shown in Figure 2B for a phosphate buffer system. Once enabled and calibrated, the Auto•Blend Plus technology will display gradient information in an intuitive manner related to pH and ionic strength (salt) as shown in Figure 3. This affords users the flexibility to investigate the effect of pH and/or salt using this single buffer system for efficient method scouting.
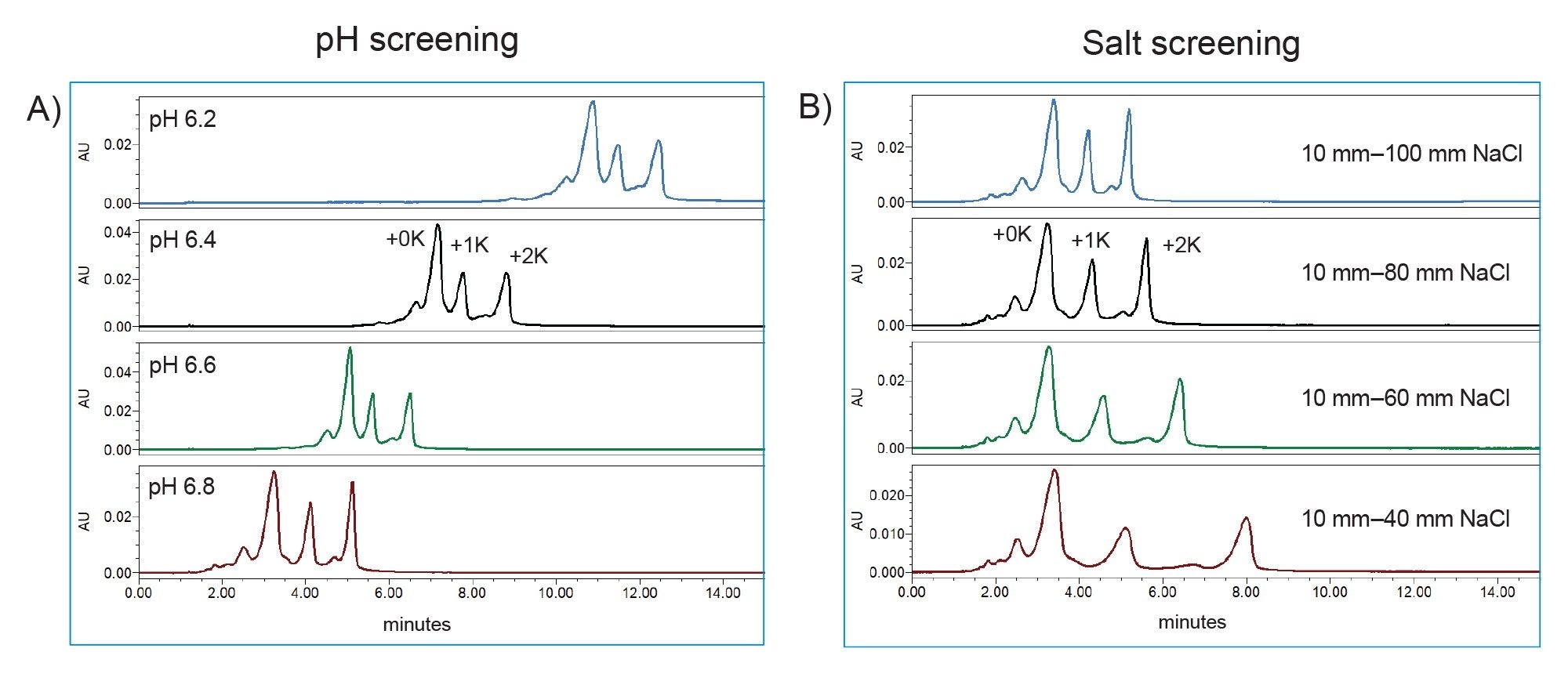
To demonstrate this principle in a practical setting, the charge variant profile of Infliximab was evaluated at different pH values using a quick screening gradient from 10–100 mM NaCl. As shown in Figure 4A, the Auto•Blend Plus method was able to scout pH values in an incremental fashion using a single reservoir buffer preparation. Using this approach, it was quickly determined mobile phases above pH=6.5 offered increased efficiency in terms of the chromatographic space used as well as offering acceptable resolution of charge variants.
Using a similar approach with the same buffer system, shallower salt gradients were then evaluated while maintaining the mobile phase at a constant pH of 6.8. As shown in Figure 4B, shallower gradients were observed to increase resolution between the main lysine charge variants (+0K, +1K, +2K). The negligible change in resolution between acidic species and the +0K lysine variant indicates the early eluting species were not strongly adsorbed to the stationary phase and were minimally impacted by the shallower salt gradients suggesting a lower mobile phase pH may be more appropriate to optimize this aspect of charge variant analysis. This observation/correlation is noteworthy in that it may have otherwise been overlooked using traditional method development practices where time constraints and instrument availability may limit the number of conditions that can be evaluated.
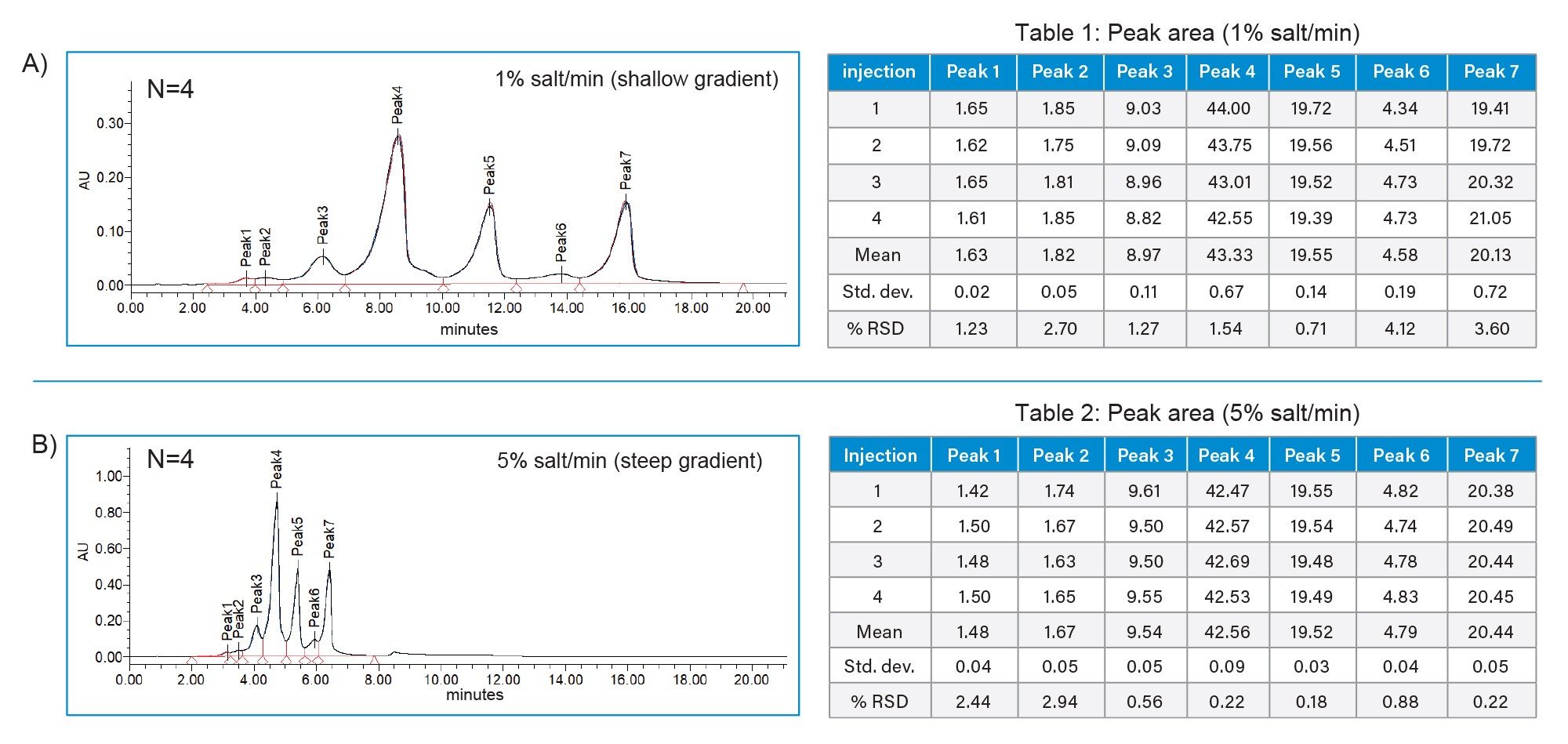
Using the knowledge gained from the initial method scouting, further optimization using the same buffer system found a mobile phase pH=6.5, in conjunction with an elevated temperature of 40 °C to facilitate use of a higher flow rate of 0.750 mL/minute, allowed for good use of the chromatographic space with acceptable resolution between charge variants when a gradient from 10–45 mM NaCl was applied (Figure 5A). Looking at the accompanying data (Table 1), it is evident the ACQUITY Premier System was able to deliver these results consistently and reproducibly given the low peak area %RSD observed for the replicate injections.
Lastly, gradient slope was investigated to see if consistent results could be achieved with higher throughput to accommodate the efficiency needs associated with manufacturing and higher throughput development environments. Using the same buffer system, gradient slopes from 1% NaCl/minute to 5% NaCl/minute were evaluated in 1% increments with gradient time proportionately reduced for each run. As shown in Figure 5B and its accompanying data (Table 2), the ACQUITY Premier System was able to maintain peak areas within 1% with a high degree of reproducibility (%RSD ≤ 3%), while reducing total assay run times from 51 minutes to 23 minutes, demonstrating the potential to support high throughput environments.
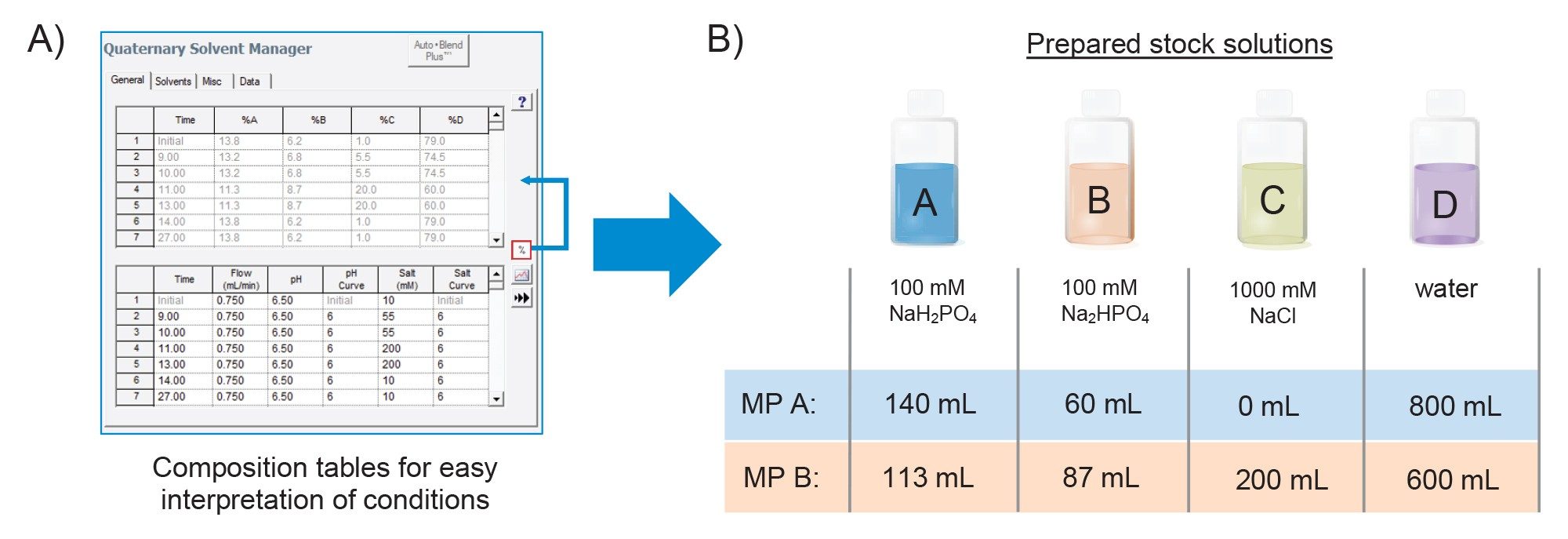
Regulated environments such as manufacturing require that methods undergo qualification, validation, and method/technology transfer, and some organizations using alternative LC platforms may not have access to software features such Auto•Blend Plus or even quaternary-based LC systems. In this consideration, Auto•Blend Plus technology allows users to access the composition table generated from its algorithm to facilitate method migration. An example of the composition table, which can be accessed from the instrument method window, is shown in Figure 6A.
To demonstrate such broader applicability, the current Auto•Blend Plus developed method was converted into a method compatible with a binary mixing mobile phase system. To do this, four freshly prepared stock solutions were prepared as shown in Figure 6B. Using the composition table reservoir percent volume, 1 L of mobile phase A was prepared with 140 mL NaH2PO4 (stock A), 60 mL Na2HPO4 (stock B), 0 mL (stock C), and 800 mL (stock D) to make a 20 mM phosphate buffer without additional NaCl. The pH was experimentally determined to be pH=6.55 which was within 0.05 of the expected pH. Similarly, mobile phase B was prepared using line four in the composition table where the stock volumes used are listed in Figure 6B. The pH of mobile phase B was experimentally determined to be pH=6.45. The good agreement between the expected and experimental pH for each mobile phase using the composition tables demonstrates that Auto•Blend Plus is capable of delivering accurate mobile phase formulations while remaining transparent in terms of compositional parameters.
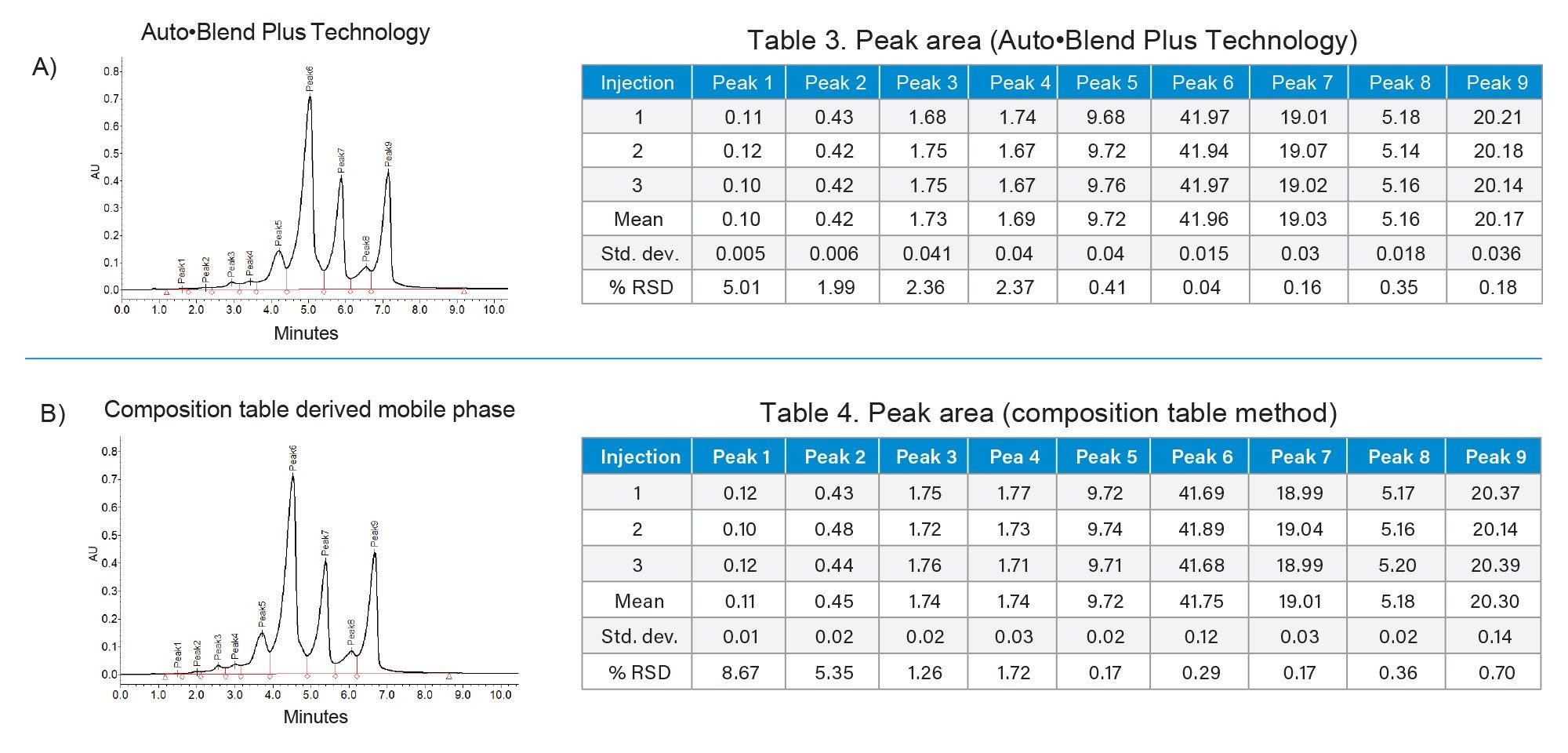
This is demonstrated in Figure 7 where both the Auto•Blend Plus method as well as the manual binary mixing method derived from the composition table were compared. As shown, both methods demonstrate consistent charge variant profiles. The slight difference in absolute retention time may be attributed to the minor pH differences where the binary mobile phase system cannot correct for the common ion effect. However, this was observed to have negligible impact on the results as both methods reported mean peak area percentage values within 0.25%. These results demonstrate that the Auto•Blend Plus method can easily be converted to a traditional method to facilitate qualification, validation, and tech transfer with minimal overhead. Collectively, this study demonstrates the value and utility Auto•Blend Plus technology offers to produce optimized methods, while reducing the time and costs associated with the development and manufacture of biotherapeutic drug products.
Conclusion
As a standard capability of the ACQUITY Premier UPLC System, Auto•Blend Plus reduces the burden and costs associated with method development process by allowing multiple buffer compositions to be tested from a single set of simple components. Access to composition tables enables users to migrate and validate methods that meet regulatory guidance with minimal effort. These features, which are fully integrated across Waters CDS/informatics solutions, makes the ACQUITY Premier System a flexible platform that can readily support the development and manufacture of biopharmaceutical drug products.
References
- Fekete et al. Ion-exchange chromatography for the characterization of biopharmaceuticals. J. of Pharmaceutical and Biomedical Analysis. 2015; 113:43–55.
- Du et al. Chromatographic analysis of the acidic and basic species of recombinant monoclonal antibodies. mAbs. 2012 Sep–Oct; 4(5):578–85.
- Eyer B et al. How Similar Is Biosimilar? A Comparison of Infliximab Therapeutics in Regard to Charge Variant Profile and Antigen Binding Affinity. Biotechnol J. 2019 Apr;14(4).
- Jung et al. Physicochemical characterization of Remsima. mAbs. 2014;6(5):1163–77.
720007603, April 2022