
This application note demonstrates the determination of perchlorate in drinking water using Single Quadrupole Mass Spectrometry.
The analysis of perchlorate anion in drinking water is a current concern for the US Environmental Protection Agency (EPA), and environmental scientists. The current EPA Method 314 using ion chromatography with suppressed conductivity detection has difficulty reliably quantifying less than 1 ppb ( μg/L) perchlorate in water containing high concentrations of dissolved solids, mainly the chloride and sulfate salts. Conductivity detection cannot provide this capability, which has caused false positive perchlorate quantification. Hence, the EPA has validated 2 chemistries all using mass spectrometry to overcome this problem. In February 2005, the official reference dose (RfD) for perchlorate was established at 0.0007 mg/kg/day. For a 70 kg person, this represents consuming 2 liters of drinking water containing 25.5 ppb perchlorate. But since perchlorate comes from many sources, sub-ppb detection limits are desirable.
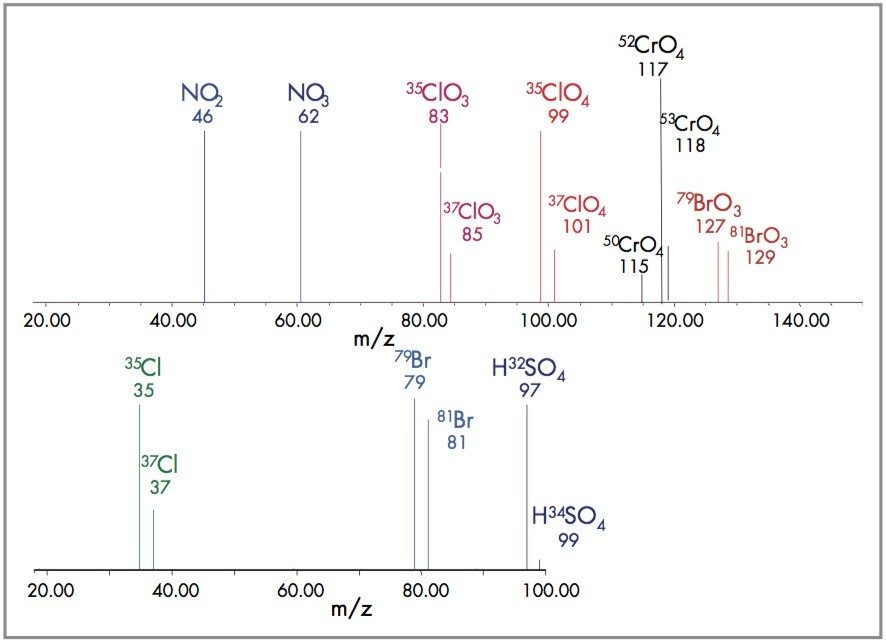
Mass spectrometry detects inorganic anions using negative electrospray (-ESP) based upon the molecular weight of the anion, Ma-. Simultaneously, several anions, such as perchlorate, are present as isotopic analogs. As an example, chlorine is found in nature approximately as 75% 35Cl and 25% 37Cl; perchlorate is present as 35ClO4-, molecular weight 99; and 37ClO4-, molecular weight 101.
The EPA Method 331 is an LC-MS/MS method not requiring chemical suppression technology, and EPA Method 332 is an LC-MS method requiring chemical suppression for the analysis of perchlorate. This application note combines the best of both methods for the simple LC-MS analysis of perchlorate.
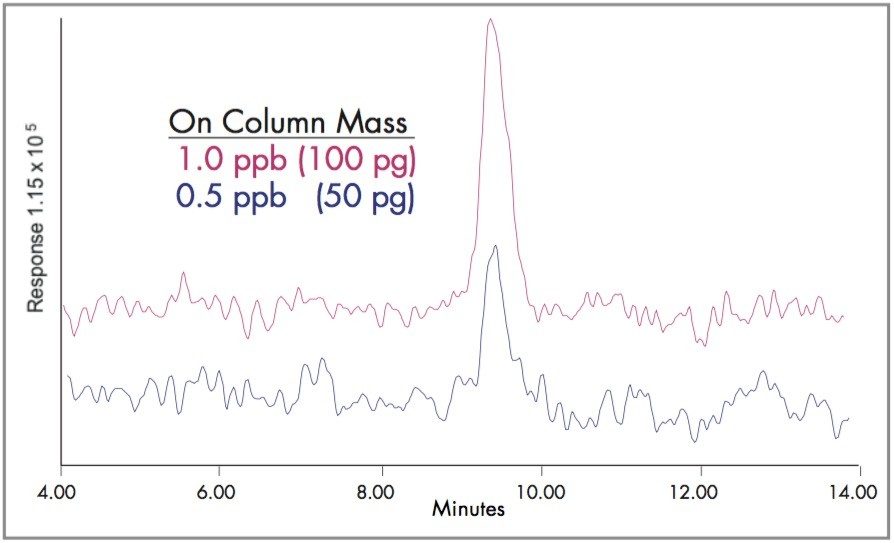
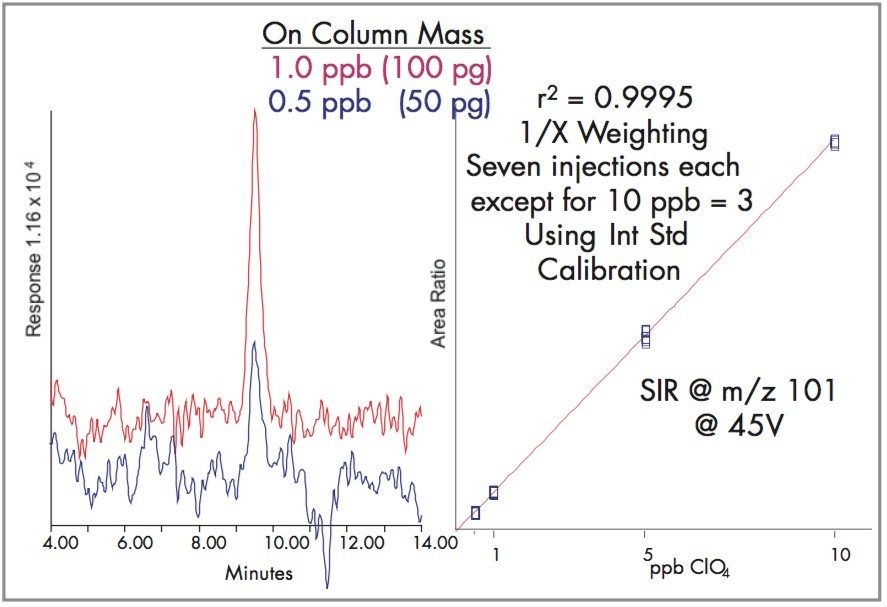
Although not as sensitive as tandem quadrupole, Waters Micromass ZQ Single Quadrupole Mass Spectrometer has demonstrated the ability for quantifying perchlorate below 1 ppb. Figure 1 shows the response of 1 ppb and 0.5 ppb perchlorate using Single Ion Recording (SIR) analysis at molecular weight 99.
Drinking water is a unique matrix varying in the concentrations of organic and inorganic salts. These high-dissolved water salts pose the greatest challenge to the IC method 314.0, and also pose a minor problem for mass spectrometry. The first is the resolution of low ppb perchlorate from high ppm concentrations of Cl and SO4.
Generally, monovalent perchlorate elutes from an anion exchange column after a divalent sulfate. This is because perchlorate has some non-polar characters that allow it to retain on the polymeric anion exchange column by reversed-phase principles. All aqueous IC eluents show this selectivity. However, if acetonitrile is added to the buffer eluent, perchlorate can be positioned to elute after high chloride, but before high sulfate. The LC TIC chromatogram of 1 ppb in a high dissolved solids matrix is shown in Figure 2.
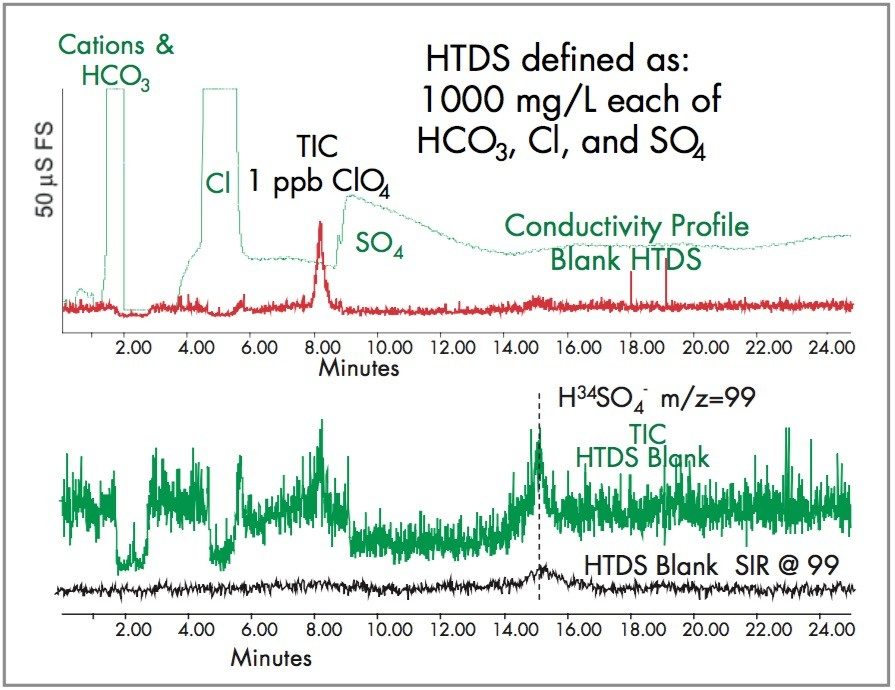
EPA Methods 331.0 and 332.0 have evaluated the response of perchlorate in varying ionic strength water and noted that as the ionic strength, or its’ conductivity increases, the response for perchlorate decreases. This suppression effect is attributed to the perchlorate partially coeluting with high sulfate where the sulfate suppresses the perchlorate response. Since sample sulfate concentration will vary, the use of an isotopically labeled (Cl18O4) perchlorate as an internal standard corrects for this problem.
The other concern about sulfate coelution is the presence of 34SO4, present at 4.2% abundance. During the -ESP ionization process, 34SO4-2 is detected as H34SO4-1, which has the same MW as perchlorate 99, shown in Figure 2. Thus, if sulfate and perchlorate partially coelute, the accuracy and ion ratio confirmation of perchlorate will be biased.
Using the LC conditions described in Table 1 and adding the labeled perchlorate internal standard, the SIR at m/z 99 calibration is linear from 0.5 to 10 ppb as shown in Figure 3, and for SIR at m/z 101 as shown in Figure 4.
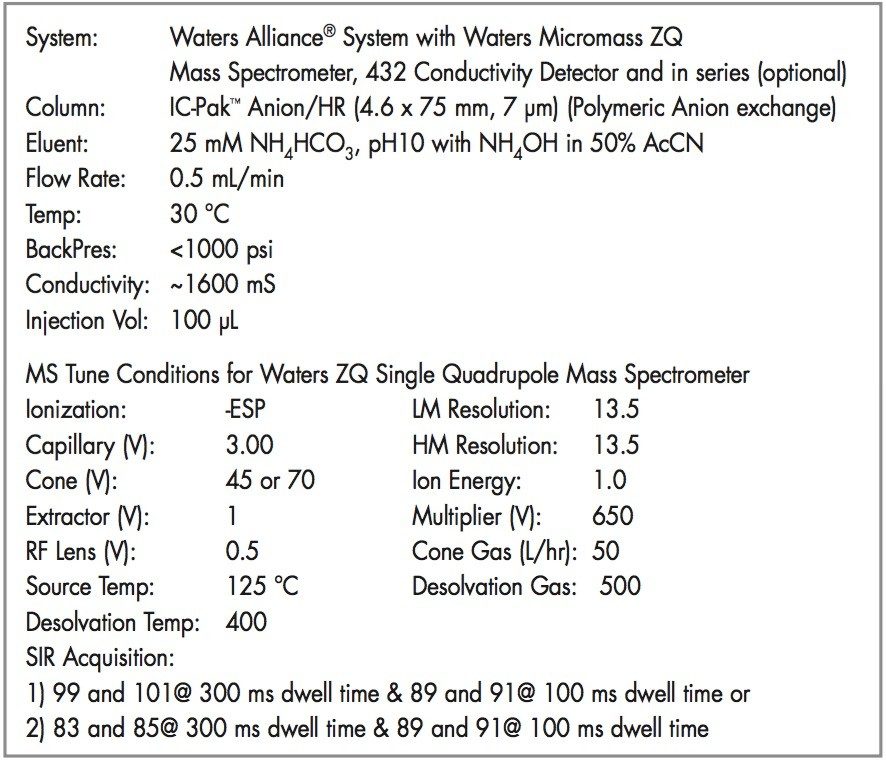
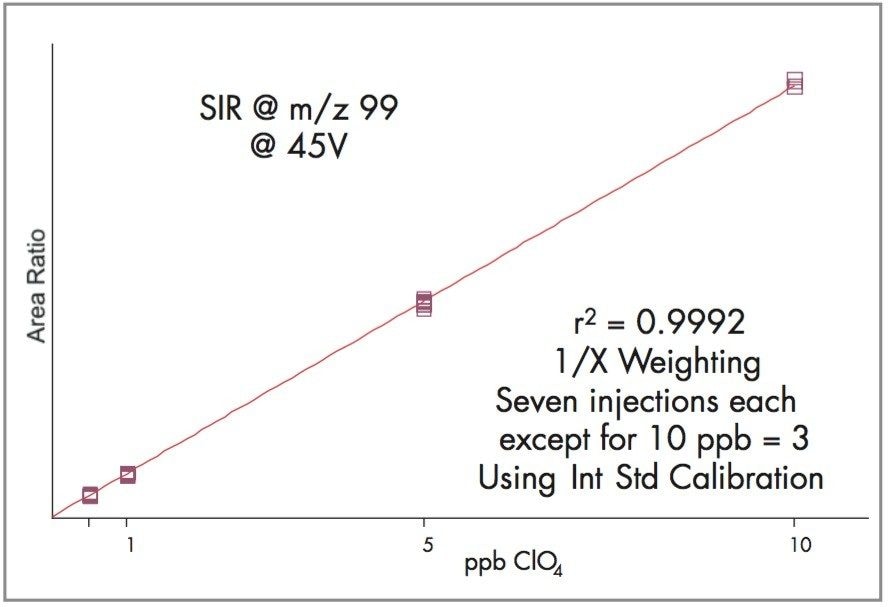
Data was acquired and processed using Waters Empower Software.
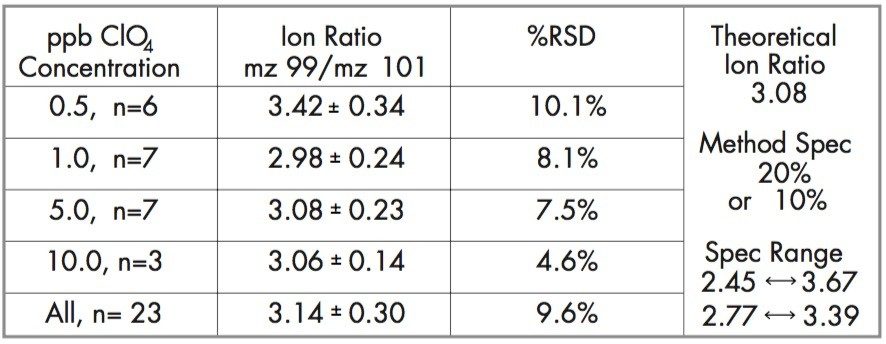
Since perchlorate comes in 2 isotopic forms (35Cl and 37Cl), m/z 99 and m/z 101, the peak area ratio of natural perchlorate compares to the labeled internal standard, m/z 107 and 109. If the ion ratios m/z 99 divided by m/z 101 are within 3.08 ± 20% of each other, then the analyte is confirmed as perchlorate. See Table 2.
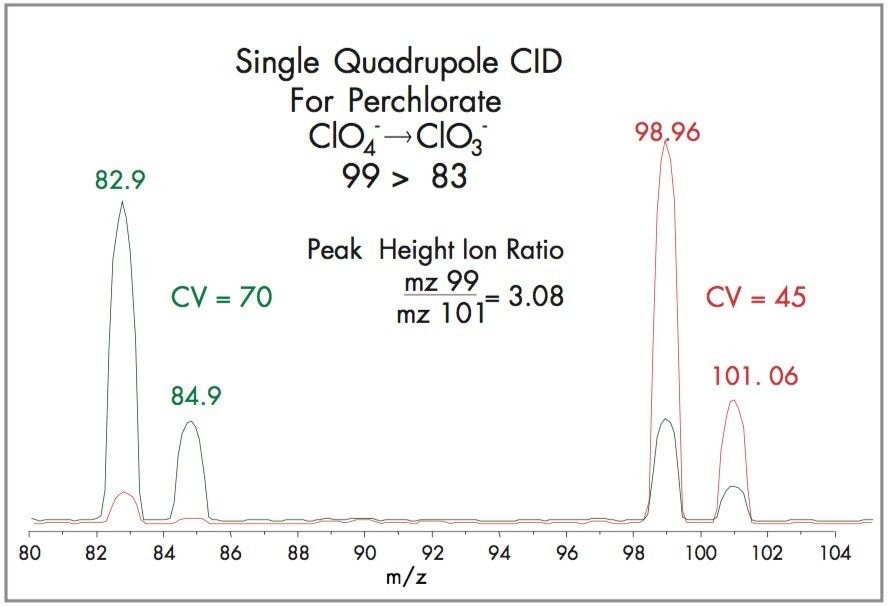
An alternative means for perchlorate confirmation uses In-Source CID, which fragments perchlorate, m/z 99, to chlorate, m/z 83, by loss of an oxygen. Perchlorate and chlorate are well resolved such that SIR at 83 will show 2 peaks, the earlier eluting chlorate followed by perchlorate.
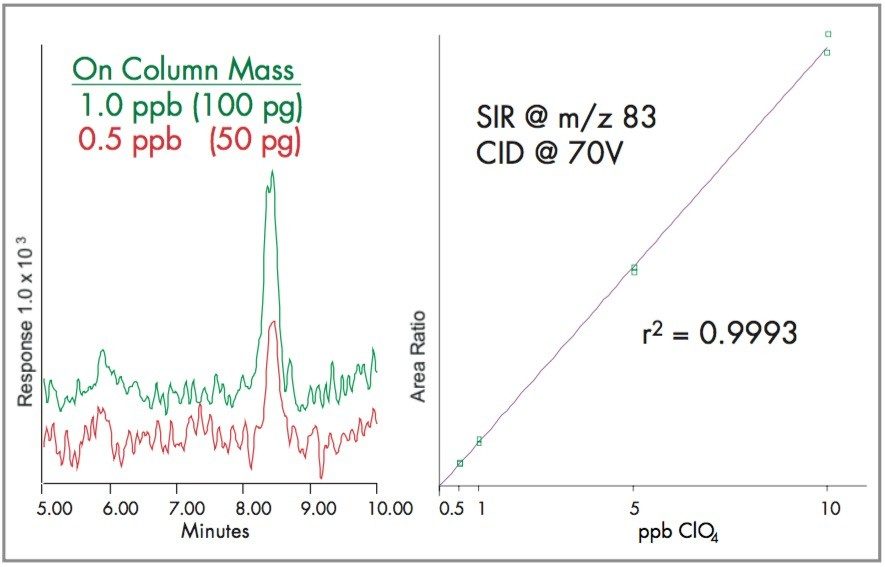
This is achieved by increasing the cone voltage to 70V. Figure 6 shows that as the cone voltage increases, the perchlorate 99 and 101 decrease, and their fragments at m/z 83 and 85 respectively increase. SIR at m/z 83 is linear and has a detection limit of 0.5 ppb.
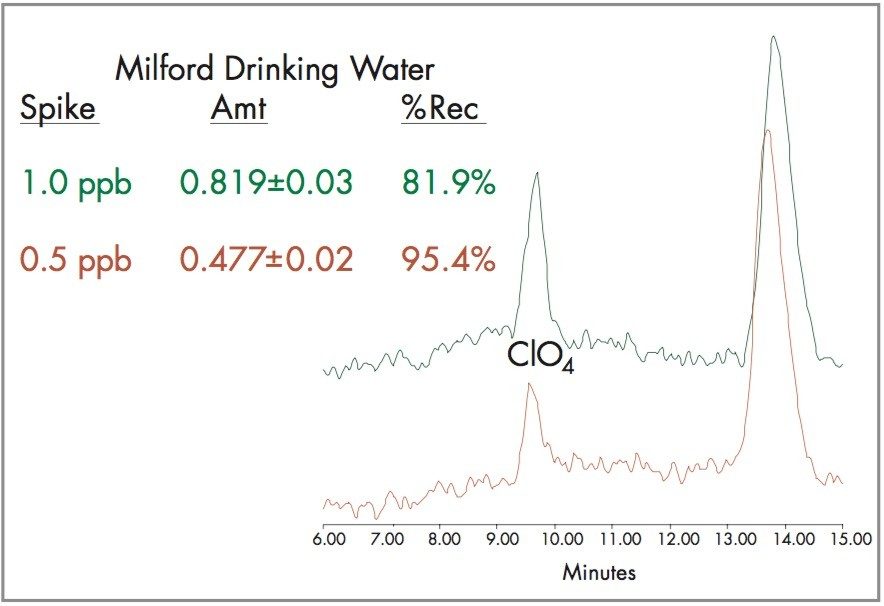
Milford, Massachusetts drinking water, a typical chlorinated drinking water, was spiked with 0.5 and 1.0 ppb perchlorate. The spiked drinking water chromatograms shown in Figure 7 indicate recoveries of 82% for the 1 ppb spike, and 95% for the 0.5 ppb spike. The data indicates that the Waters ZQ Single Quadrupole Mass Spectrometer can be used for EPA Methods 331.0 and 332.0 with the ability to meet the required <1 ppb perchlorate detection limit.
During the method development process, other common anions were added to the water to see if their presence would effect perchlorate response. With this eluent, perchlorate, NO2, NO3 and PO4 elute in the same region. Figures 8 and 9 show the chromatography of various inorganic ions with their isotopic ratio. Neither the regulated anions nor the common anions found in drinking water interfere with perchlorate, which allows this method to be used in a high total dissolved solids matrix.
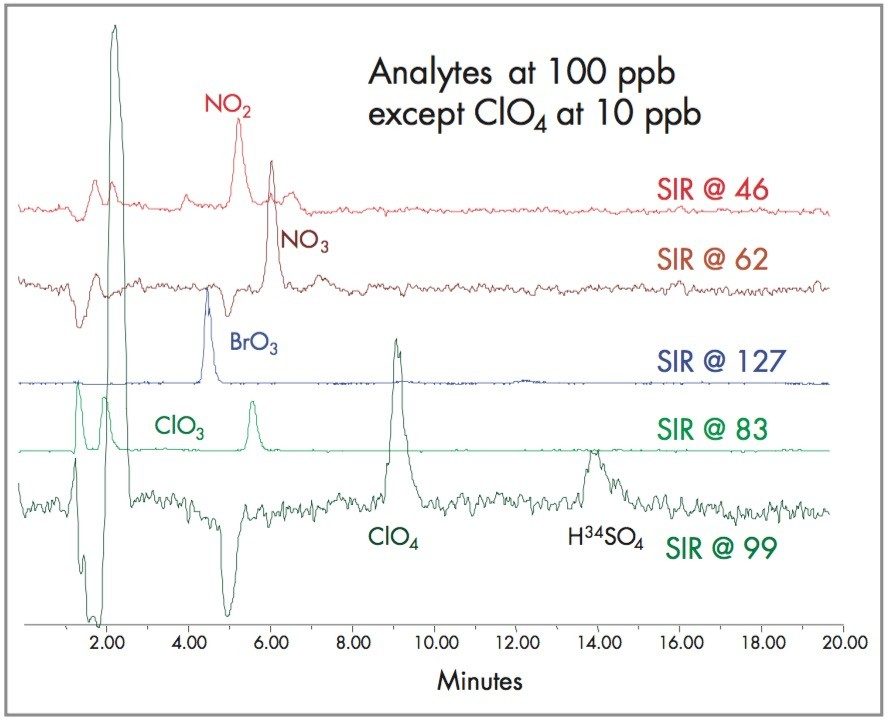
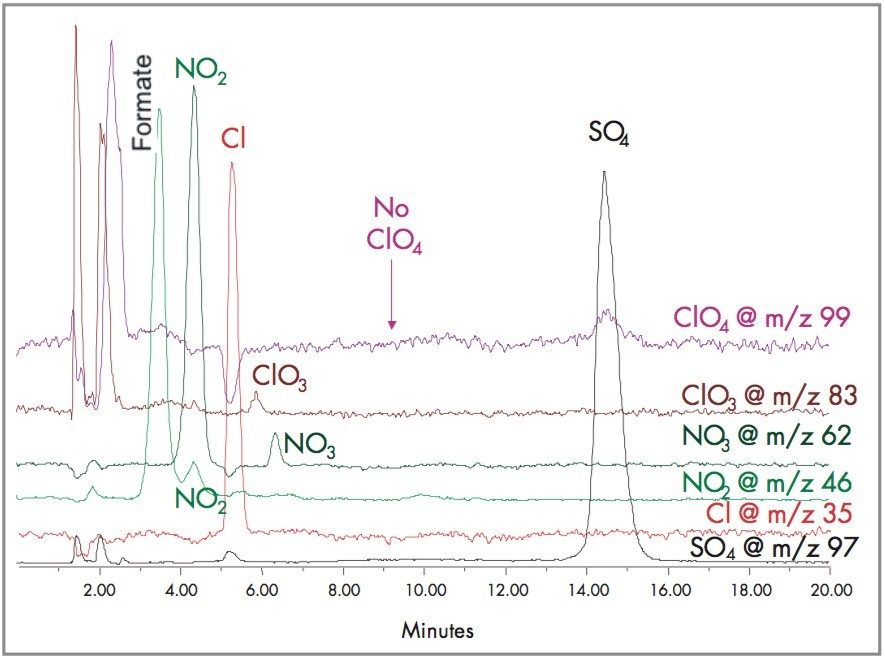
These SIR chromatograms demonstrates the feasibility of using LC-MS to analyze for the other regulated anions, bromate, chlorite, chlorate, perchlorate, nitrite, nitrate and chromate in a single analysis. Figure 10 shows the inorganic profile of a chlorinated drinking water.
720001285, August 2005