For forensic toxicology use only.
The method presented in this study demonstrates the use of Ostro Sample Preparation Plates combined with UPLC-MS/MS for the analysis of 22 opioid compounds and metabolites of interest in whole blood samples. All compounds were analyzed in less than 5.5 minutes with complete resolution of all isobaric compound pairs. The use of Ostro Sample Preparation Plates allowed for rapid, in-well cell lysis and protein precipitation, followed by elution into a 96-well collection plate. This procedure resulted in improved throughput compared to protein precipitation in individual tubes, with the added benefit of removing endogenous phospholipid compounds. All analytes demonstrated good linearity over the entire calibration range, and the method was sensitive for reliable detection at the lowest curve points.
The analysis of natural and synthetic opioid drugs continues to be an important aspect of forensic toxicology. A substantial percentage of arrests and/or deaths are attributed to the misuse or abuse of narcotic pain relievers such as oxycodone and hydrocodone, as well as the illegal opiate, heroin. Forensic laboratories often need to analyze whole blood specimens for the presence of different drugs to determine the precise cause of death, in cases of driving under the influence of drugs, or other criminal or research purposes. In the past, opioid analyses were typically conducted by GC/MS after first subjecting the samples to acid or enzymatic hydrolysis to liberate glucuronide metabolites.1 This step of using enzymatic hydrolysis to convert glucuronide metabolites to their free form adds time and expense to analysis, and complete and consistent hydrolysis is not always assured.2 With the advent of modern UPLC-MS/MS techniques, glucuronide metabolites can now be analyzed directly.3-6
Many sample preparation strategies have been used for whole blood analysis, including liquid-liquid extraction (LLE) and solid phase extraction (SPE). One of the simplest involves cell lysis followed by protein precipitation. The method presented in this study describes a rapid and straightforward sample preparation strategy using Ostro Sample Preparation Plates whereby whole blood samples can be pre-treated to lyse the cells, precipitated with acetonitrile, and eluted using a simple 96-well format. All sample pre-treatment is conducted within the wells of the Ostro Plate, without the need for centrifugation or sample transfer from individual tubes.
Following sample preparation, 22 opioid drugs and metabolites are subsequently analyzed by UPLC-MS/MS. Glucuronide metabolites are directly analyzed, eliminating the need for enzymatic or chemical hydrolysis. Calibration curves are linear with appropriate limits of detection easily reached.
|
System: |
ACQUITY UPLC |
|
Column: |
ACQUITY UPLC BEH C18 2.1 x 100 mm, 1.7 μm (p/n 186002352) |
|
Column temp.: |
30 °C |
|
Injection volume: |
10 μL |
|
Flow rate: |
0.4 mL/min |
|
Mobile phase A: |
0.1% formic acid in MilliQ water |
|
Mobile phase B: |
0.1% formic acid in ACN |
|
Gradient: |
Initial conditions were 2% B. The %B was increased to 52.8% over 6 minutes, then returned to 2% over 0.5 minute. The system was allowed to re-equilibrate for 1.5 minutes. The entire cycle time was 8.0 minutes. |
|
Mass spectrometer: |
Xevo TQD |
|
Ionization mode: |
ESI positive |
|
Acquisition mode: |
MRM (see Table 1 for transitions) |
|
Capillary voltage: |
1 kV |
|
Collision energy (eV): |
Optimized for individual compounds (see Table 1) |
|
Cone voltage (V): |
Optimized for individual compounds (see Table 1) |
Waters MassLynx Software v4.1
All compounds and internal standards (IS) were purchased from Cerilliant (Round Rock, TX). Complementary, deuterated internal standards were used for all compounds with the exception of hydromorphone-3-glucuronide, codeine- 6-glucuronide, and norbuprenorphine-glucuronide. For these compounds, a deuterated IS with the most similar response was chosen as a surrogate.
A combined stock solution of all compounds (10 μg/mL; 2.5 μg/mL for fentanyl) was prepared in methanol. Working solutions were prepared daily with high standards and QCs in matrix (blood) and performing serial dilutions to achieve the desired concentrations. Calibrator concentrations ranged from 5 to 500 ng/mL for all analytes except fentanyl, which was prepared at 25% of the concentration of the other analytes (1.25 to 125.00 ng/mL). A combined internal standard stock solution (5 μg/mL; 1.25 μg/mL for fentanyl) was prepared in ACN.
Whole blood samples were prepared by adding 150 μL of aqueous 0.1 M ZnSO4/0.1 M NH4CH3COOH to the wells of an Ostro Pass-through Sample Preparation Plate. 50 μL of whole blood was added to the ZnSO4/NH4CH3COOH solution and mixed briefly (5 s) to lyse the cells. 600 μL of ACN containing internal standards was then added to the prepared samples. After vortexing for 3 minutes, the samples were eluted into a 96-well collection plate, evaporated to dryness under N2, and reconstituted in 50 μL of 0.1% formic acid in 2% ACN. 10 μL was injected onto the LC-MS/MS system.
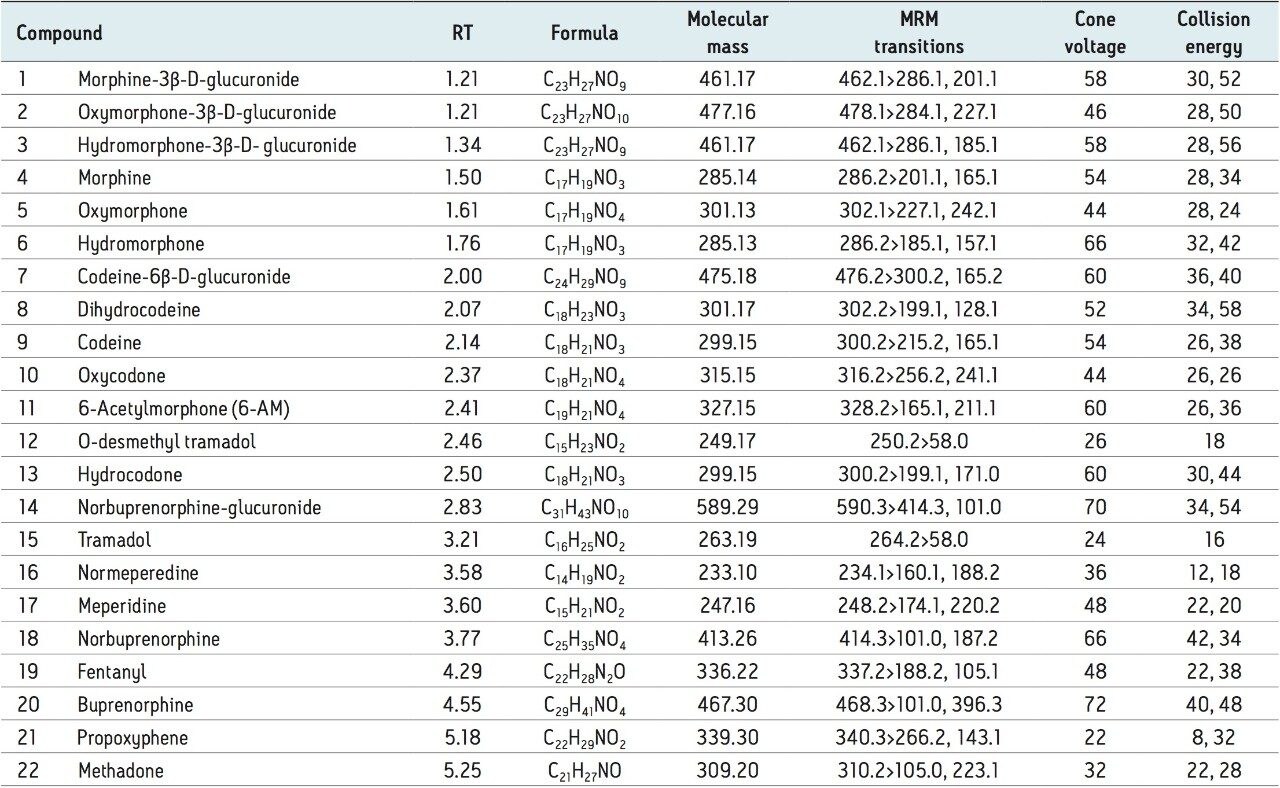
The 22 compounds and metabolites screened, listed in Table 1, constitute a comprehensive panel of natural opiate drugs, semi-synthetic opioids, and synthetic narcotic analgesic compounds. Most of the compounds are weak bases, with pKa values of approximately 8 to 9. They have a wide range of polarities, with LogP values ranging from -3.48 for morphine-3β-d-glucuronide to 5.0 for methadone. MRM transitions used are also listed in Table 1. With the exception of tramadol and O-desmethyl tramadol, primary and confirmatory MRM transitions are listed along with their respective collision energies.
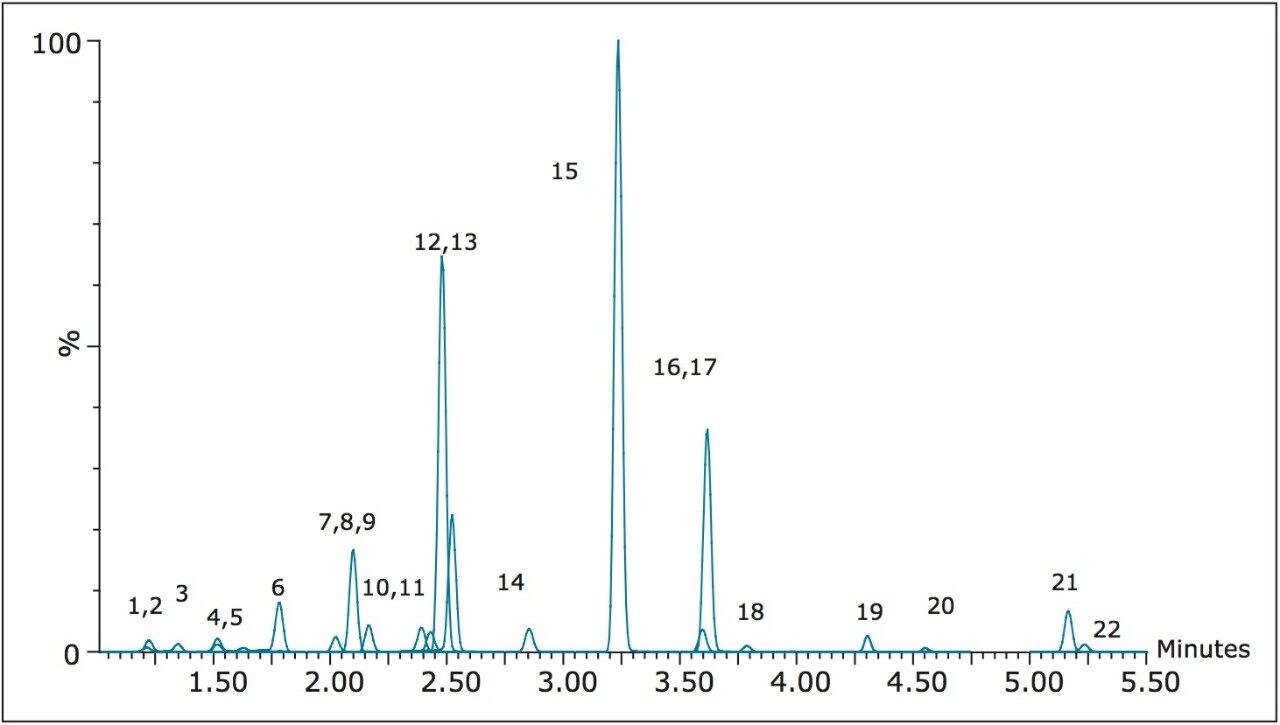
A representative chromatogram of all compounds from a 50 ng/mL calibration standard is shown in Figure 1. Peak assignments can be found in Table 1. Using an ACQUITY UPLC BEH C18 2.1 x 100 mm, 1.7 μm Column, all analytes were analyzed in less than 5.5 minutes with a baseline separation between the critical isomer pairs of morphine-3-glucuronide and hydromorphone-3 glucuronide (compounds 1 and 3), morphine and hydromorphone (compounds 4 and 6), and codeine and hydrocodone (compounds 9 and 13). Total cycle time was 8.0 minutes.
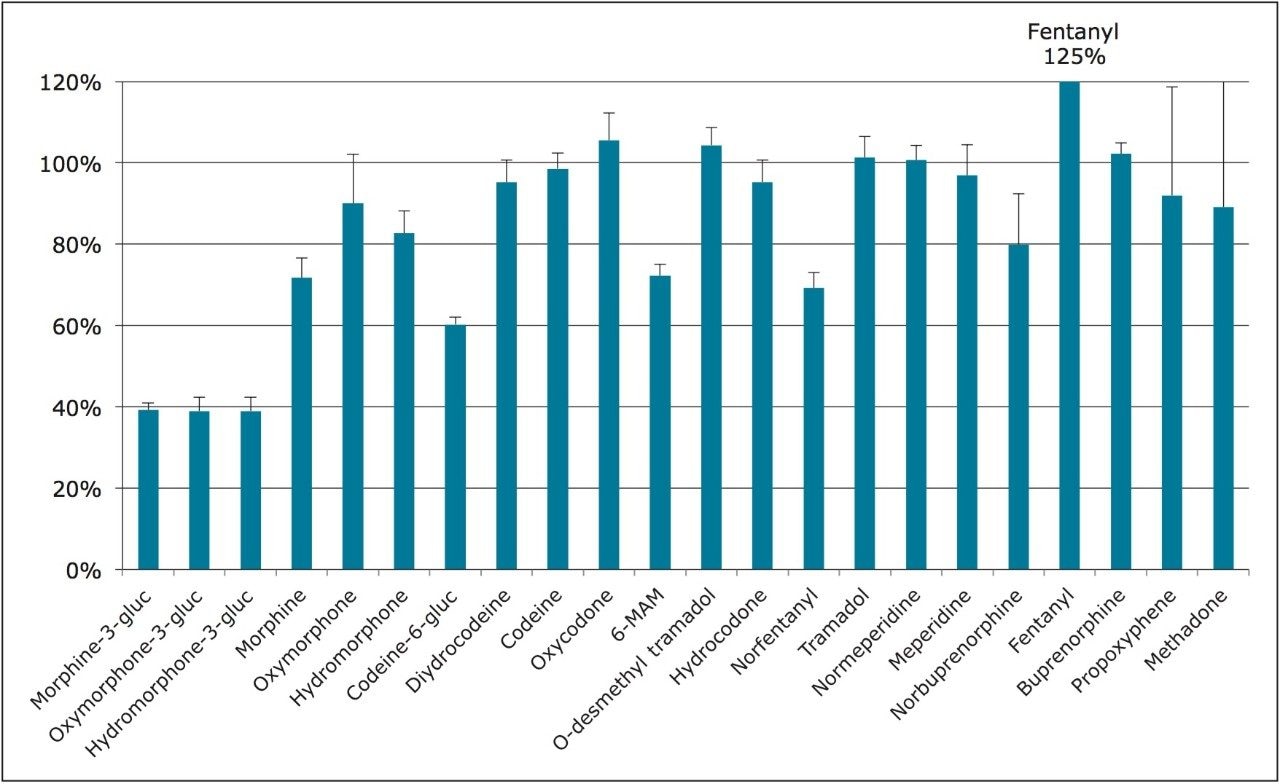
For this application, evaporation of the organic eluate and reconstitution in a high aqueous solution (2% ACN) was necessary to prevent solvent effects that, otherwise, would interfere with the chromatography of the glucuronide metabolites. Figure 2 shows the average recovery of all compounds from whole blood using the Ostro Pass-through protocol detailed above. With the exception of the three earliest eluting glucuronide metabolites, all compounds demonstrated recoveries of 60% or greater, and the majority of compounds were recovered at 80% or greater.
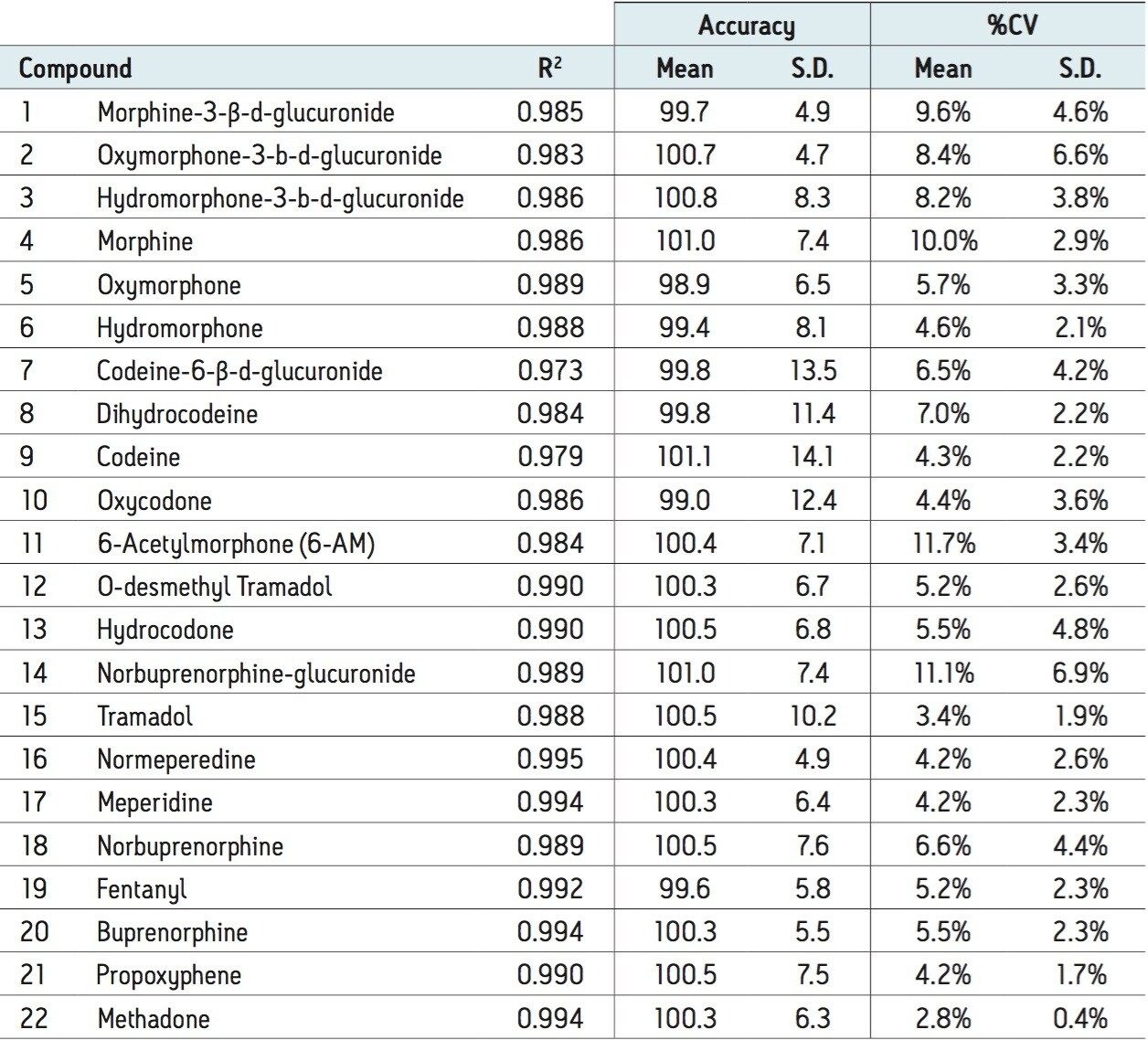
The whole blood extraction method described herein was evaluated for linearity. Calibration standards were prepared in urine at concentrations ranging from 5 to 500 ng/mL (1.25 to 125.00 ng/mL for fentanyl). Table 2 summarizes the R2 values, % deviations, and %CVs for all of the compounds. The average deviation of all calibration points from the curve was less than 2% for all compounds, and mean %CV values were less than 10% for all but three compounds. Individually, >93% of all calibrators were within 15% of their nominal concentration and >95% of the %CVs for individual calibration points (N=3) were less than 15%. Individual R2 values were all greater than 0.973, and all but 2 were greater than 0.98. In addition to the good linearity seen over the calibration range, all compounds demonstrated excellent sensitivity. At 5 ng/mL, the UPLC-MS/MS signal for oxymorphone-3-glucuronide, the least sensitive compound, was at least 20x greater than that of a blank extracted whole blood sample. In most cases, the signal for this low calibrator well exceeded 100x the method blank signal.
The method presented in this study demonstrates the use of Ostro Pass-through Sample Preparation Plates combined with UPLC-MS/MS for the analysis of 22 opioid compounds and metabolites of interest in whole blood samples. All compounds were analyzed in less than 5.5 minutes with complete resolution of all isobaric compound pairs. The use of Ostro Pass-through Sample Preparation Plates allowed for rapid, in-well cell lysis and protein precipitation, followed by elution into a 96-well collection plate. This procedure resulted in improved throughput compared to protein precipitation in individual tubes, with the added benefit of removing endogenous phospholipid compounds. All analytes demonstrated good linearity over the entire calibration range, and the method was sensitive for reliable detection at the lowest curve points.
720004673, November 2014