
For forensic toxicology use only.
This application note details the extraction of cocaine, benzoylecgonine and cocaethylene from urine samples using Oasis MCX μElution Mixed-mode Cation Exchange Plates for forensic toxicology.
The water wettability of this sorbent enabled the elimination of conditioning and equilibration steps, simplifying the extraction procedure and speeding up the sample preparation workflow, without sacrificing performance. In addition, the low elution volume eliminated the need for evaporation and reconstitution of the final eluate.
Cocaine is a powerfully addictive stimulant that directly affects the brain. Cocaine stimulates key pleasure centers by preventing the reuptake of monoamine neurotransmitters including dopamine, norepinephrine, and serotonin, increasing the concentration and persistence of these neurotransmitters within synapses.1 As of 2008, it was estimated that approximately 21.9 million people in the United States had used cocaine in the past month.2 Although its prevalence has been decreasing slightly, it is still one of the most widely used illegal drugs. Thus, there is a need for accurate analysis of cocaine and its metabolites by facilities such as crime and forensic labs, pain management research laboratories, or anti-doping agencies. A modern, UPLC MS/MS method could be a substantial improvement over GC-MS or older HPLC-MS methods, enabling faster and more reliable results.
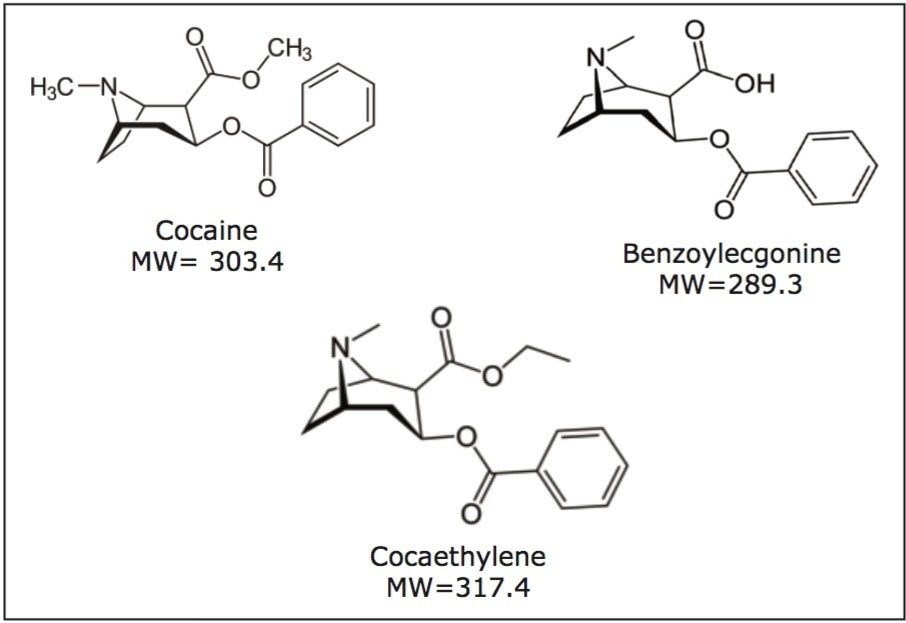
Cocaine can be detected in urine for 12–72 hours depending on the dose. But one of its metabolites, benzoylecgonine (BZE), has a half life that can be double that of cocaine, enabling a longer detection window. Therefore, in cocaine users, benzoylecgonine testing in urine is often a more reliable indication of cocaine use than the parent drug.3 Cocaethylene (CE), is a psychoactive ethyl homologue “metabolite” of cocaine, and is formed exclusively during the co-administration of cocaine and alcohol,4 and also remains in the system longer than cocaine. Figure 1 shows the structures of cocaine, benzoylecgonine and cocaethylene.
This application note details the sample extraction and UPLC-MS/MS analysis of cocaine and two of its metabolites in urine using Waters’ Oasis MCX (mixed-mode, reversed phase/strong cation-exchange) μElution Plate. The water wettability of the sorbent enabled direct loading of samples onto the sorbent, eliminating traditional conditioning and equilibration steps and streamlining the workflow. Additionally, the reliable performance of Xevo TQD offers excellent sensitivity and linearity, enabling reliable quantification.
All standards and stable isotope labelled internal standards were purchased from Cerilliant (Round Rock, TX). Combined calibrator solutions of cocaine, benzoylecgonine, and cocaethylene were prepared in human urine at concentration levels ranging from 1 to 1000 ng/mL. QC Standards were prepared at concentration levels of 3, 30, and 300 ng/mL from a separate working standard. The working internal standard solution of 5000 ng/mL cocaine-d3, benzoylecgonine-d8, and cocathylene-d8 was prepared in 25% methanol. 20 μL of this was added to 200 μL of urine yielding a final concentration of 500 ng/mL.
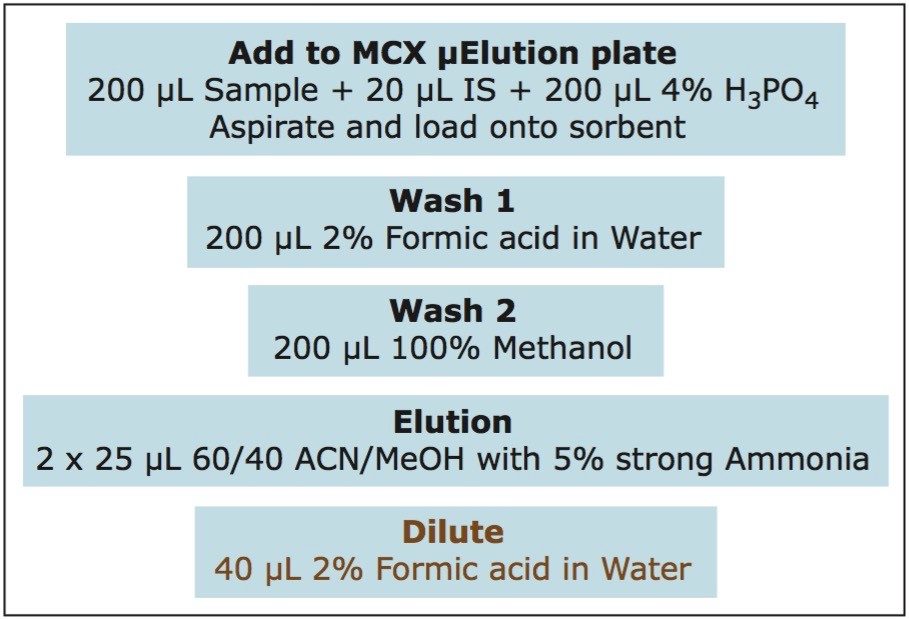
Samples were prepared as follows: 200 μL of each sample was placed into the well of the SPE plate, followed by 20 μL of the internal standard solution and 200 μL of 4% H3PO4. The samples were mixed within the well and then loaded onto the sorbent by vacuum. All wells of the SPE plate were then washed with 200 μL of 2% formic acid in water, followed by 200 μL of methanol. The samples were then eluted with 2 x 25 μL aliquots of 60:40 ACN:MeOH with 5% strong ammonia solution and diluted with 40 μL of 2% formic acid in water. 2 μL was injected onto the UPLC-MS/MS system. The sample pre-treatment and extraction procedure are summarized in Figure 2. Analyte recoveries were calculated according to the formula below:
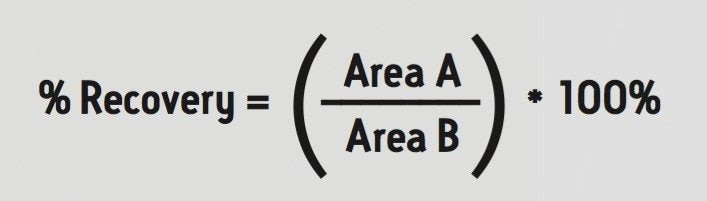
Where A = the peak area of an extracted sample and B = the peak area of an extracted matrix sample to which the compounds were added post-extraction.
|
LC system: |
ACQUITY I-Class UPLC System |
|
Column: |
ACQUITY UPLC HSS T3, 100Å, 1.8 μm, 2.1 x 50 mm |
|
Column temp.: |
30 °C |
|
Sample temp.: |
10 °C |
|
Mobile phase A (MPA): |
Water with 0.1% formic acid |
|
Mobile phase B (MPB): |
ACN with 0.1% formic acid |
|
Weak Needle Wash (WNW): |
2% ACN |
|
Strong Needle Wash (SNW): |
20% ACN |
|
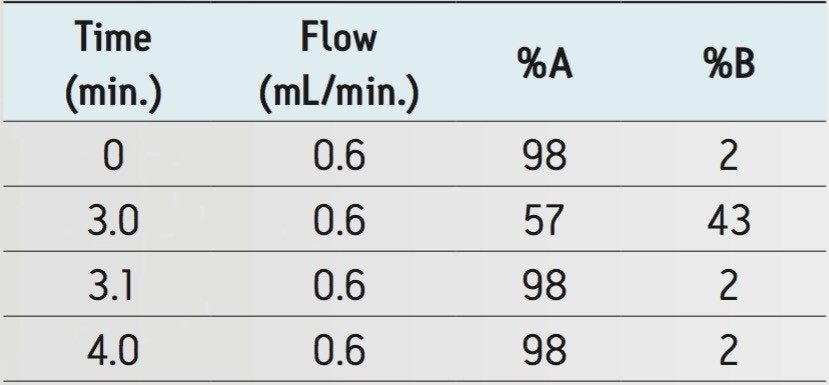
The gradient ramp is shown in Table 1. |
|
MS system: |
Xevo TQD Mass Spectrometer |
|
Ionization mode: |
ESI Positive |
|
Capillary voltage: |
0.25 kV |
|
Cone voltage: |
Optimized for each analyte |
|
Desolvation gas: |
1000 L/hr |
|
Cone gas: |
150 L/hr |
|
Desolvation temp.: |
500 °C |
|
Source temp.: |
150 °C |
Data were acquired and analyzed using MassLynx Software (v4.1). MRM transitions and MS voltages were optimized using Intellistart MS Optimization Software. Quantification was performed using TargetLynx.
Figure 3 shows chromatography of cocaine, benzoylecgonine and cocaethylene from an extracted 50 ng/mL urine sample. All compounds eluted within 3 minutes. The ACQUITY UPLC HSS T3 Column offered increased retention over other C18 columns while still maintaining peak widths of under 3 seconds.
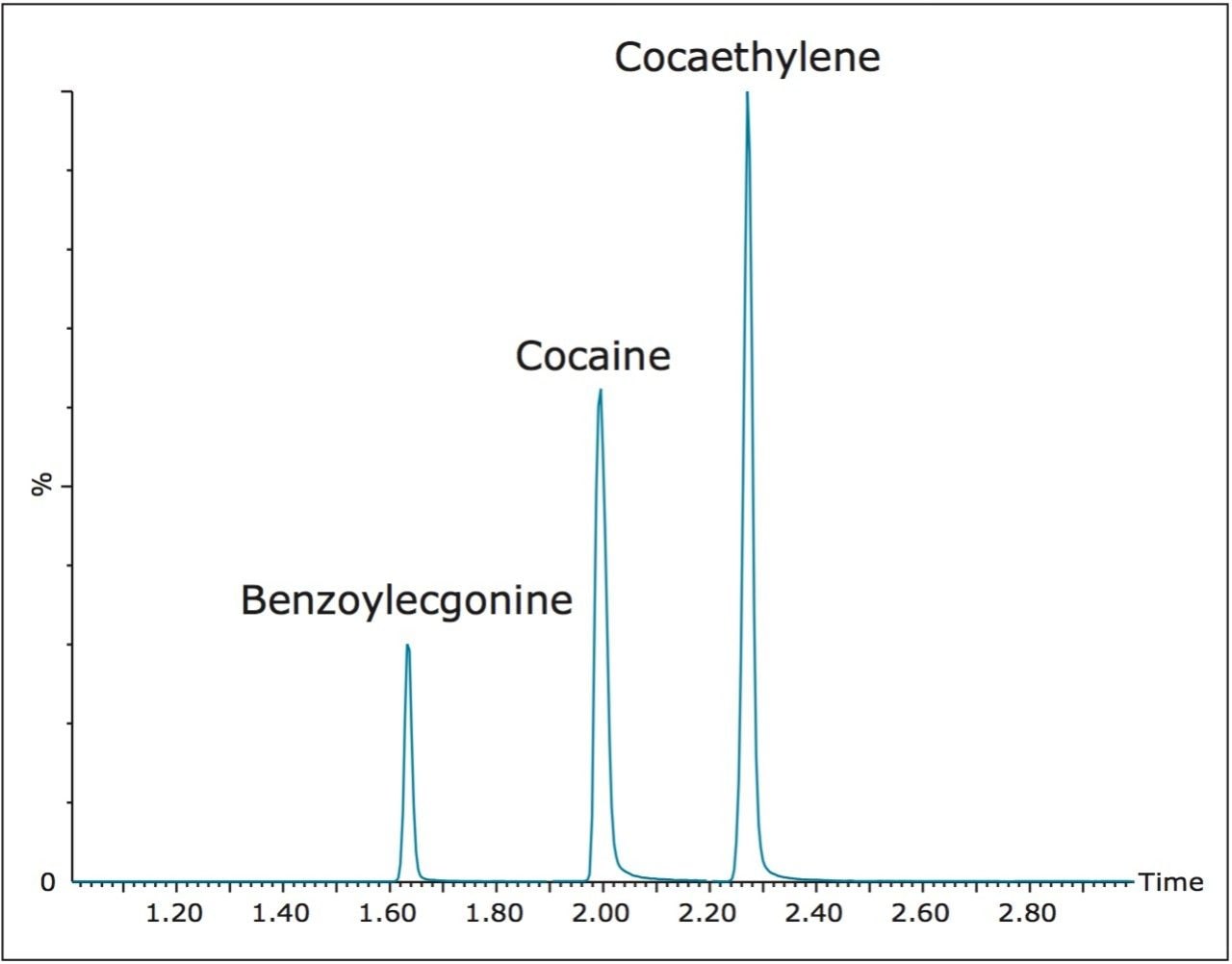
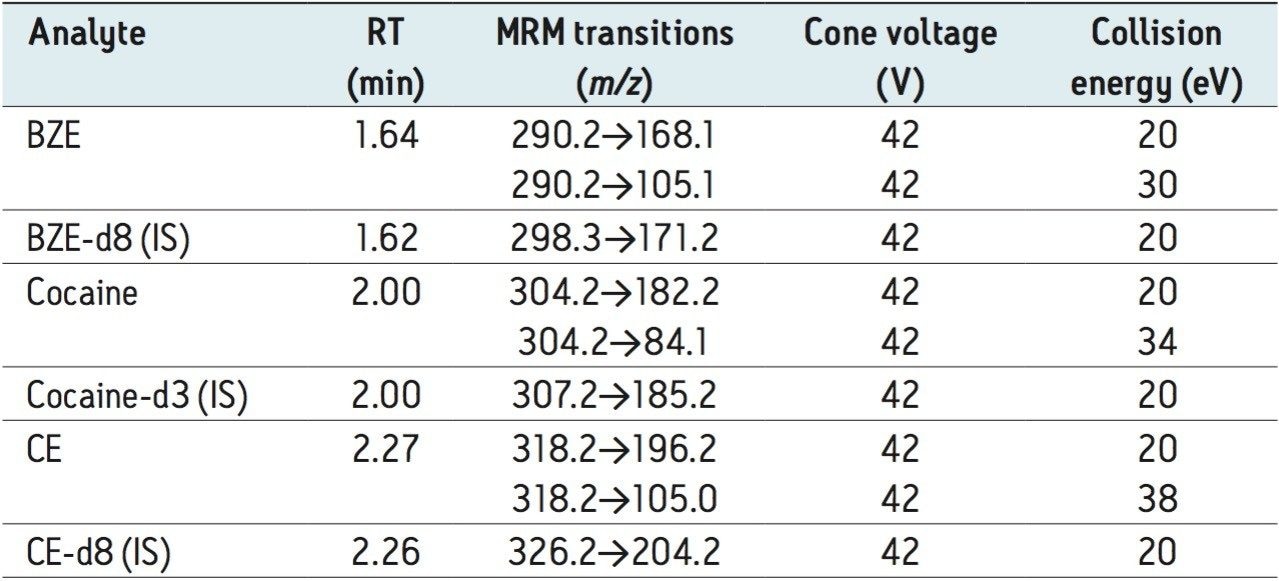
Table 2 lists the retention time and individualized MS parameters of all compounds and their stable isotope labelled internal standards, including MRM transitions, cone voltages, and collision energies. Two MRM transitions were used for each compound, a primary (listed first) and a confirmatory transition (listed second).
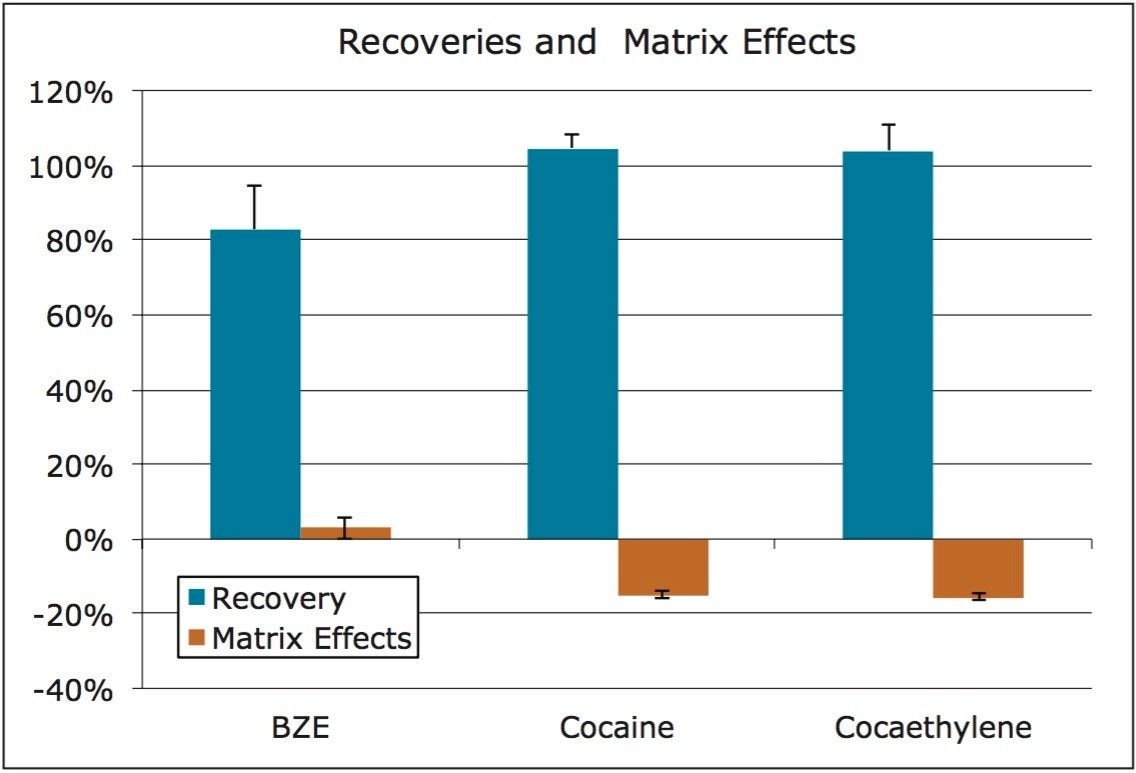
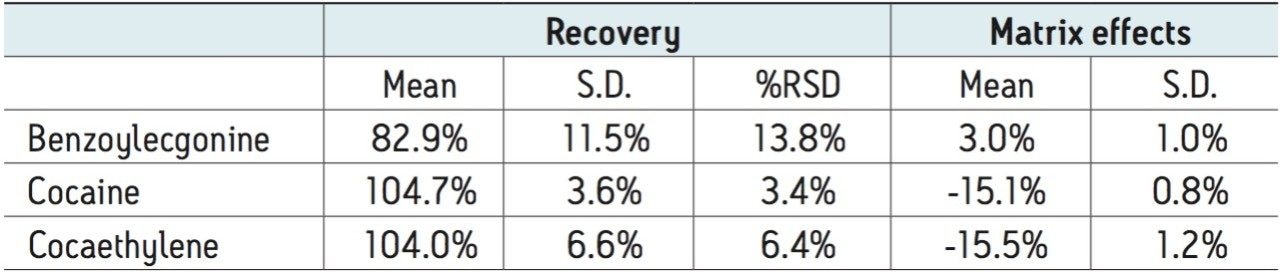
Extraction recovery was consistent for all compounds. As Figure 4 and Table 3 show, recovery for all compounds was 83–105% with low %RSDs, demonstrating the reproducibility of Oasis MCX. Matrix effects were low for all compounds, indicating minor ion suppression. The average matrix effect magnitude for all compounds was 11.2%. Once again, the low standard deviations (≤1.2%) demonstrate the consistency of extraction and cleanup seen with Oasis MCX. All recovery and matrix effect data are summarized in Table 3.
Calibration and quality control samples were prepared as previously described in the materials and method section. Calibration ranges were from 1–500 ng/mL for benzoylecgonine and from 1–1000 ng/mL for the other two compounds. Quality control samples were prepared at low, medium, and high concentrations as appropriate for the calibration ranges.
All compounds had linear responses over the entire calibration range with R2 values of 0.99 or greater with 1/x2 weighting. Table 4 summarizes the data from the calibration curves.

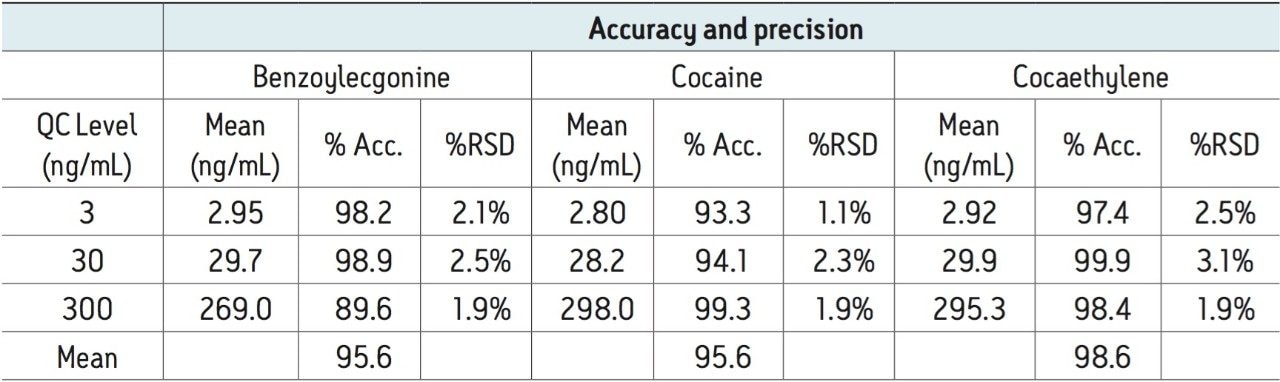
Quality control results were accurate and precise, with all results within 10% of expected values and %CVs no greater than 7% (N=6). This data can be seen in Table 5. The excellent accuracy and precision demonstrate the consistency and robustness of this method.
This application note details the extraction of cocaine, benzoylecgonine and cocaethylene from urine samples using Oasis MCX μElution Mixed-mode Cation Exchange Plates for forensic toxicology. The water wettability of this sorbent enabled the elimination of conditioning and equilibration steps, simplifying the extraction procedure and speeding up the sample preparation workflow, without sacrificing performance. In addition, the low elution volume eliminated the need for evaporation and reconstitution of the final eluate.
Recoveries were greater than 83% and were consistent for all compounds, with %RSDs under 15%. Matrix effects were less than -16% for cocaine and cocaethylene and under 3% for benzoylecgonine. Linearity, accuracy, precision and analytical sensitivities were excellent for the three compounds. All accuracies were within 11% of target concentrations and all %RSDs were less than 7% for QC samples demonstrating the high reproducibility of the extraction sorbent and the method. This rapid and simple solid phase extraction method yielding high recoveries and low matrix is coupled to a 4 minute UPLC method to provide fast and accurate support for the analysis of cocaine and metabolites.
720005571, January 2016