
For forensic toxicology use only.
This is an Application Brief and does not contain a detailed Experimental section.
Phosphatidylethanol (PEth) species are direct biomarkers of alcohol consumption that have attracted increasing interest recently due to their selectivity and relatively long half-life compared to other alcohol biomarkers. Waters has developed a rapid, clean, and robust method for the extraction of PEth 16:0/18:1 and 16:0/18:2 from whole blood that features Ostro Pass-Through Sample Preparation plates, an ACQUITY UPLC I-Class PLUS System and the Xevo TQ-S micro to achieve the performance and analytical sensitivity necessary for this method.
Phosphatidylethanol (PEth) species are abnormal phospholipids formed in cell membranes in the presence of ethanol. More than 40 homologs have been reported. However the most abundant PEth species in whole blood are 16:0/18:1 and 16:0/18:2, accounting for approximately 37% and 25% of total PEths, respectively.1 PEth is a direct marker of even moderate alcohol consumption and appears unaffected by factors such as age, gender, and liver disease. PEth can also be used to distinguish drinking patterns and behaviors, e.g., to identify moderate or excessive drinkers, as well as binge drinking. PEths have become popular markers for ethanol use recently due to their relatively long window of detection of up to 3–4 weeks vs 3–4 days for ethyl glucuronide (EtG) and ethyl sulfate (EtS) as monitored in urine. PEth also has a high degree of selectivity compared to other biomarkers and compounds such as EtG and EtS.2-5 Baseline PEth concentrations are usually <10 ng/mL (0.014 µM) in abstinent individuals and cutoffs of 0.05 µM have been suggested to indicate moderate drinking behaviors.3 Concentrations above 0.3 µM (210 ng/mL) have been suggested to indicate heavy or binge drinking.2 However, the extraction and analysis of PEth from whole blood poses some unique analytical challenges due to the extreme hydrophobicity and unique chemical nature of these molecules.
This work details a solution for the quantitative analysis of PEth that uses solid phase extraction (SPE), followed by a rapid UPLC-MS/MS method for the analysis of PEth 16:0/18:1 and PEth 16:0/18:2 from whole blood. Ostro Pass-Through Sample Preparation plates were used in a simple 2-step process (load and elute). Method optimization revealed unique, multi-modal retention characteristics that appeared to have both reversed-phase and HILIC properties. Elution on a BEH C8 Column combined with a strong mobile phase composed of 50:50 ACN:IPA resulted in a rapid method with no detectable carryover.
PEth 16:0/18:1, PEth 16:0/18:2, and the deuterated analogue PEth 16:0/18:1-D5 were obtained from Cerilliant (Round Rock, TX). PEth 16:0/18:1-D5 was used as an internal standard for both molecules. Whole blood was obtained from Lampire Biological Products (Pipersville, PA).
One hundred microliters of whole blood was precipitated in two stages. First, 200 µL of isopropanol (IPA) containing 50 ng/mL deuterated internal standard was added and vortex-mixed for 5-10 seconds to fully mix the sample. Next, 800 µL of ACN containing 0.1% formic acid (FA) was added immediately, and the samples were once again vortexed. The samples were then centrifuged at 21K rcf, for 10 min, and the supernatant was loaded directly onto Waters Ostro Pass-Through Sample Preparation plates. Samples were eluted with 2 x 400 µL aliquots of 60:20:20 ACN:IPA:water. Twenty microliters were analysed using an ACQUITY UPLC I-Class PLUS System (FTN) in combination with a Xevo TQ-S micro Tandem Quadrupole MS.
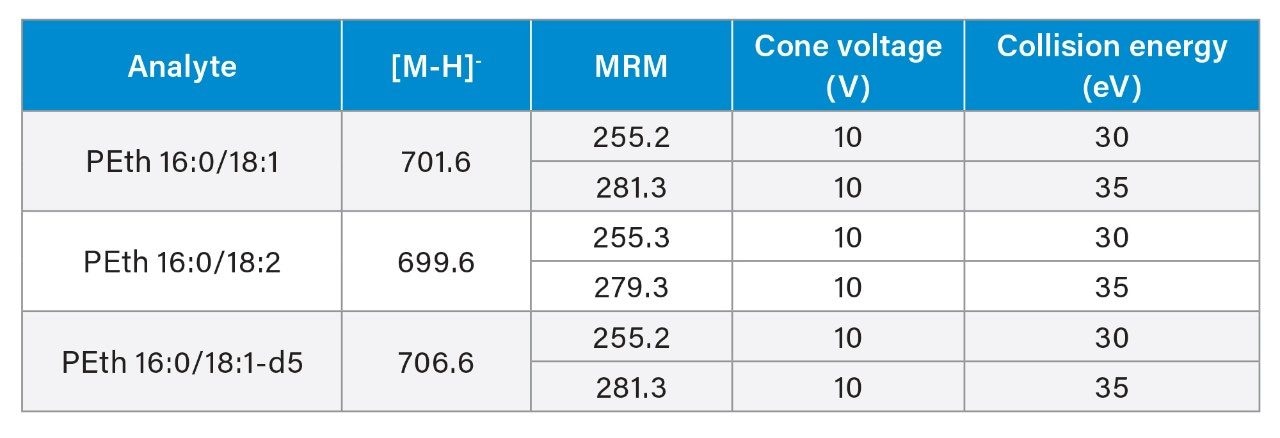
Analytes were chromatographically separated on a 1.7 µm Waters BEH C8 Column (2.1 x 50 mm) at 40 °C. Mobile phase A (MPA) was 5 mM ammonium formate with 0.1% formic acid; mobile phase B consisted of 50:50 ACN:IPA. The flow rate was 0.5 mL/min. The solvent ramp started at 50:50 MPA:MPB and increased to 100% MPB over three minutes. Two rapid 30 second ramps from 50:50 MPA:MPB to 100% MPB were added after the analytical run to minimize carryover. Samples were analyzed in negative ESI mode. Two transitions were monitored for each compound. MS parameters are listed in Table 1. Calibration curves ranged from 10–1000 ng/mL (0.014–1.4 µM). The method was validated for extraction recovery, matrix effects, linearity, accuracy, precision, analytical sensitivity, carryover, dilution integrity, and extracted sample stability.
Method optimization revealed a possible multimodal retention mechanism for PEth on the Ostro sorbent. There appeared to be some HILIC character, as a substantial proportion of water (20%) and another protic solvent (MeOH or IPA) was necessary to elute PEth off the sorbent. At the same time, strong reversed-phase elution solvents were also required due to PEth’s high lipophilicity. The use of IPA vs. methanol as a third co-solvent resulted in increased and more consistent recoveries. The final elution solvent after optimization was 60:20:20 ACN:Water:IPA.
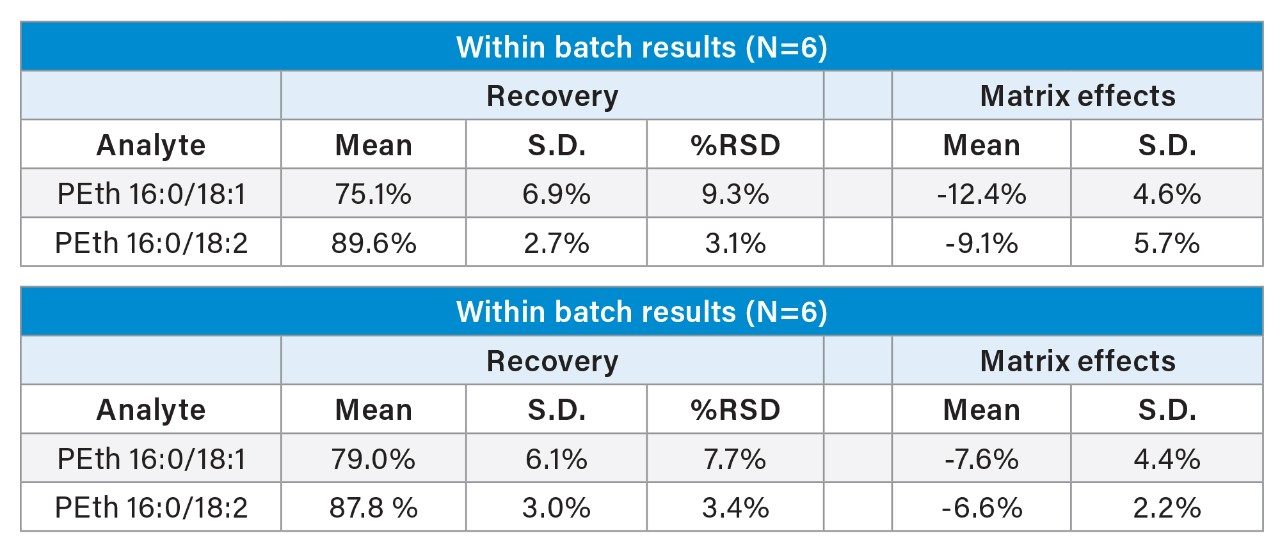
This extraction method results in high recoveries with minimal ion suppression and readily meets the analytical sensitivity requirements of the method. The extraction method was simple, efficient, and clean. PEth 16:0/18:2 and PEth 16:0/18:1 had recoveries that averaged 88% and 79%, respectively. The extraction was reproducible with all %RSDs <10%. Matrix effects were minimal at <13% for both molecules. Results for recovery and matrix effects can be seen in Table 2.
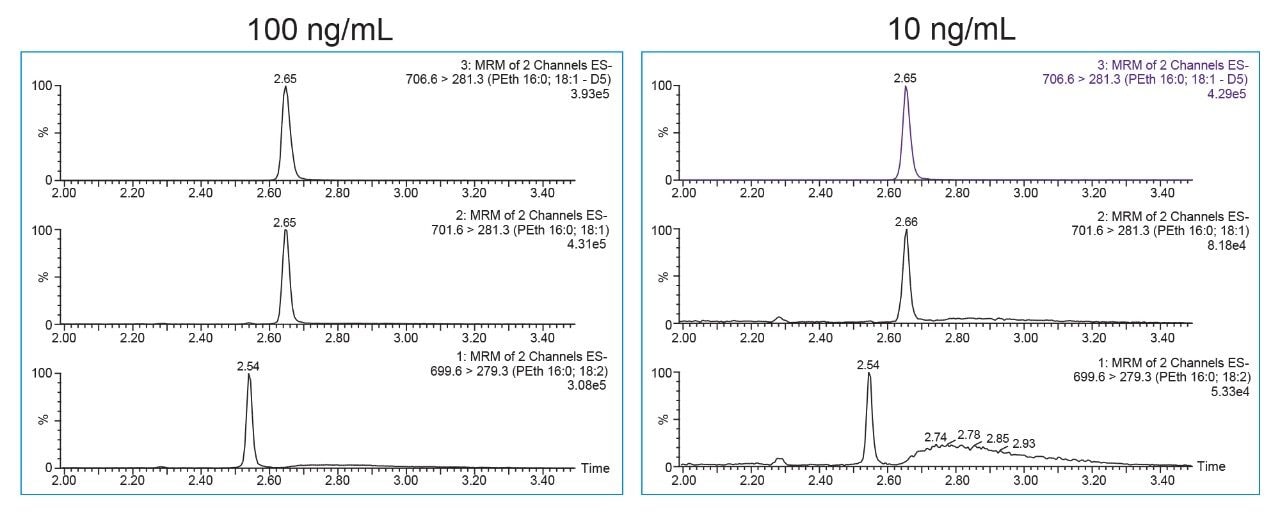
The UPLC-MS/MS method was optimized to balance the required retention and selectivity of the method while minimizing carryover. This was achieved by using the C8 Column with a strong mobile phase B consisting of 50:50 ACN:IPA. Initial method development indicated that a C18 Column was too retentive and mobile phase consisting of ACN only was not strong enough to adequately elute PEth and prevent carryover. In addition to the mobile phase optimization, a rapid “sawtooth” gradient was used after the analytical ramp to further minimize the potential for carryover. Using the conditions described here, PEth 16:0/18:1 and PEth 16:0/18:2 were baseline separated from one another, and from other interfering substances in whole blood. This can be seen in Figure 1 by the broad peaks that elute after PEth 16:0/18:1 and PEth 16:0/18:2. Retention times for PEth 16:0/18:2 and 16:0/18:1 were 2.54 and 2.64 min, respectively. No detectable carryover was seen even after injection of the high standard (1000 ng/mL).
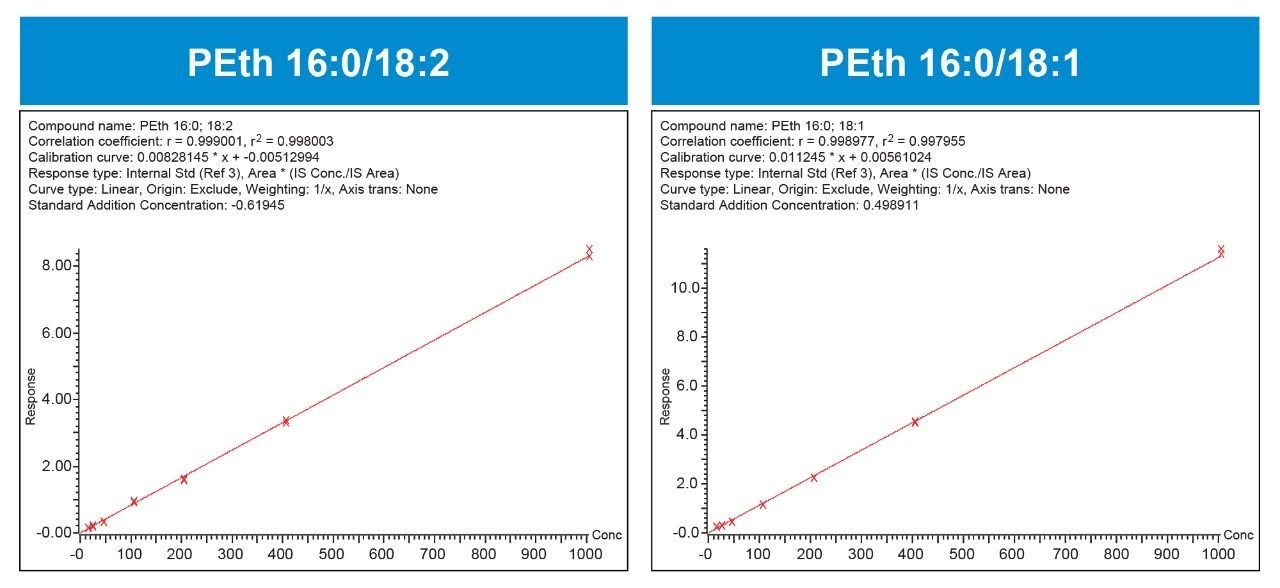
The analytical sensitivity of the method was more than adequate, with limits of quantification of 10 ng/mL (0.014 mM) for both molecules, easily meeting method requirements and limits of detection reported in the literature.2,3 Although there was a small existing contribution of PEth in the blood matrix (~5 ng/mL), method validation revealed that the method could readily distinguish and accurately quantify the addition of 10 ng/mL at the lowest calibration level. The method was linear from 10 to 1000 ng/mL (0.014–1.4 μM). Calibration curves for PEth 16:0/18:1 and PEth 16:0/18:2 are shown in Figure 2.
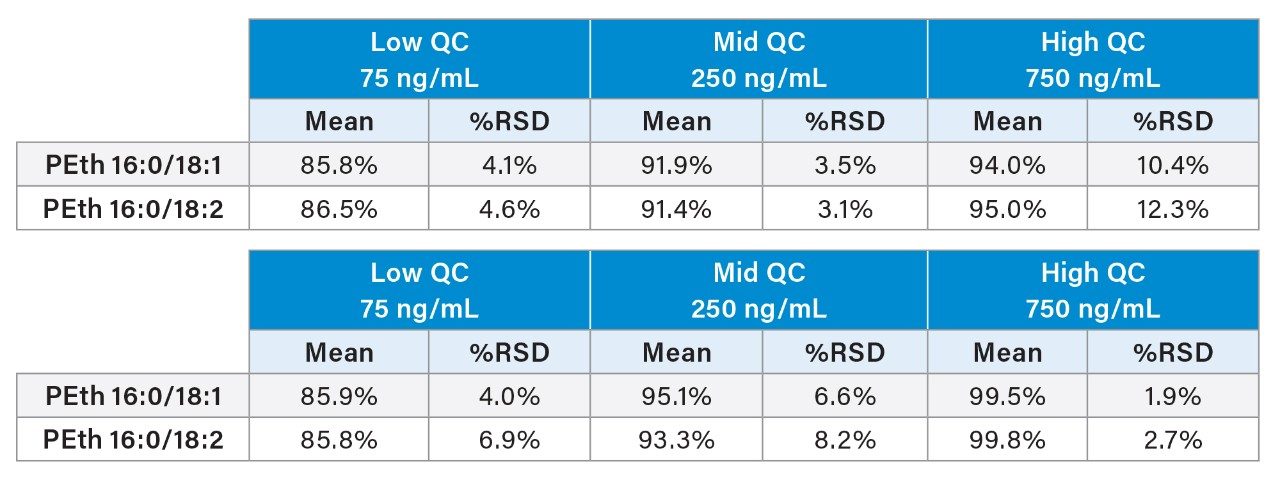
Accuracy and precision results are shown in Table 3. All accuracies with within 15% of target values and most were under 10%. All %RSDs were under 13% with most under 10%. The limit of quantitation was defined as the lowest calibrator (10 ng/mL) and confirmed during validation. Accuracy was within 15% and %RSD was 5.1%.
We have developed a bioanalytical method for the quantitative analysis of PEth 16:0/18:1 and PEth 16:0/18:2 in whole blood. The method uses protein precipitation followed by a simple extraction using Ostro Pass-Through Sample Preparation plates. Analysis using the Waters ACQUITY UPLC System and Xevo TQ-S micro Mass Spectrometer resulted in a method that was linear, accurate and precise, had no detectable carryover, and had the analytical sensitivity necessary to easily meet the suggested cutoff levels of 0.05 µM.
720007120, January 2021