This application note presents a fast, efficient method for the analysis of food dyes in a variety of foods and beverages using the ACQUITY UPLC H-Class System with an ACQUITY UPLC Photodiode Array (PDA) extended wavelength (eλ) Detector. This 10-minute method, which includes column wash and equilibration time, allows for the simultaneous analysis of 15 artificial food dyes and selected isomers.
Artificial color additives are widely used to enhance the appearance of food and beverages. Since the visual aspect of a product can be an important factor in a consumer’s decision, food dyes are often added to compensate for the loss of natural color that can occur during processing and storage. Synthetic dyes are frequently chosen over natural dyes because of certain advantages including better stability, brightness, lower cost and availability of a range of colors. However, because of concerns about the potential health risks from the consumption of artificial food dyes, synthetic colorants are subject to regulation.
Food safety data has been evaluated through toxicological studies to determine the potential effects on human health and acceptable daily intake of these additives. Global regulations can vary as to which dyes are allowed, specific foods they can be used in, and regulatory limits. For example, the FDA allows the use of Tartrazine (FD&C Yellow 5), Sunset Yellow (FD&C Yellow 6), Brilliant Blue (FD&C Blue 1), and Allura Red (FD&C Red 40) which are commonly used in the United States, along with Indigo Carmine (FD&C Blue 2), Fast Green FCF (FD&C Green 3), and Erythrosine (FD&C Red 3) which are less commonly used.1 In the EU, color additives such as Patent Blue V and Green S are allowed at certain levels only in specified food products. In order to meet these varying regulatory requirements and to ensure food safety, accurate and efficient analysis methods are needed.
Many different methods have been employed for the determination of synthetic dyes in food and beverages including thin layer chromatography and capillary electrophoresis. However, these methods can be time consuming and may not be applicable for the simultaneous analysis of many dyes. Conventional HPLC methods have been employed for the analysis of synthetic colorants and while useful, these methods require long analysis times and large amounts of expensive solvents.
This application note presents a fast, efficient method for the analysis of food dyes in a variety of foods and beverages using Waters ACQUITY UPLC H-Class System with an ACQUITY UPLC Photodiode Array (PDA) extended wavelength (eλ) Detector. This 10-minute method, which includes column wash and equilibration time, allows for the simultaneous analysis of 15 artificial food dyes and selected isomers. The combination of ACQUITY UPLC and PDA detection provides the accuracy and sensitivity necessary to ensure food safety and quality while decreasing analysis time and solvent use, and increasing throughput.
A variety of drink mixes, popsicles, powdered dessert mixes, jelly beans, hard candy, jams, and smoked salmon were purchased from a local supermarket. Powders were dissolved in water and diluted as necessary. The popsicles were either injected directly or diluted with water as necessary. The candy was dissolved in water until all color was removed or the candy was completely dissolved, and centrifuged. For the jam samples, 10 g were weighed and extracted with 50 mL water, sonicated for 15 minutes, and centrifuged. 3 g smoked salmon samples were homogenized in a blender. Then, 10 mL of a 80:1:19 ethanol:ammonia:water solution was added and the solution was shaken for 30 minutes with a Hand Motion Shaker (part # EL680.Q). The sample was then centrifuged for 10 minutes and analyzed.2,3
Artificial dye standards were purchased from Sigma Aldrich. The dyes were dissolved in water to create individual standards, and then combined into standard mixes ranging from 0.5 to 10,000 ppb. The standards were analyzed in triplicate.
|
UPLC system: |
ACQUITY UPLC H-Class |
|
Runtime: |
10 min |
|
Column: |
ACQUITY UPLC BEH C18 1.7 μm, 2.1 x 100 mm |
|
Mobile phase A: |
10 mM ammonium acetate in water |
|
Mobile phase B: |
Methanol |
|
Mobile phase C: |
Acetonitrile |
|
Flow rate: |
0.45 mL/min |
|
Injection volume: |
10 μL |
|
Time (min) |
A (%) |
B(%) |
C(%) |
|---|---|---|---|
|
Initial |
97 |
2 |
1 |
|
1.4 |
97 |
2 |
1 |
|
1.6 |
90 |
9 |
1 |
|
7.4 |
50 |
25 |
25 |
|
7.5 |
2 |
49 |
49 |
|
8.5 |
2 |
49 |
49 |
|
8.6 |
97 |
2 |
1 |
|
10.0 |
97 |
2 |
1 |
|
Detector: |
ACQUITY UPLC PDA eλ |
|
Wavelength range: |
210 to 700 nm |
|
Extracted wavelengths: |
400, 500, and 630 nm |
|
Resolution: |
3.6 nm |
|
Sampling rate: |
20 pts/sec |
|
Filter time constant: |
Normal |
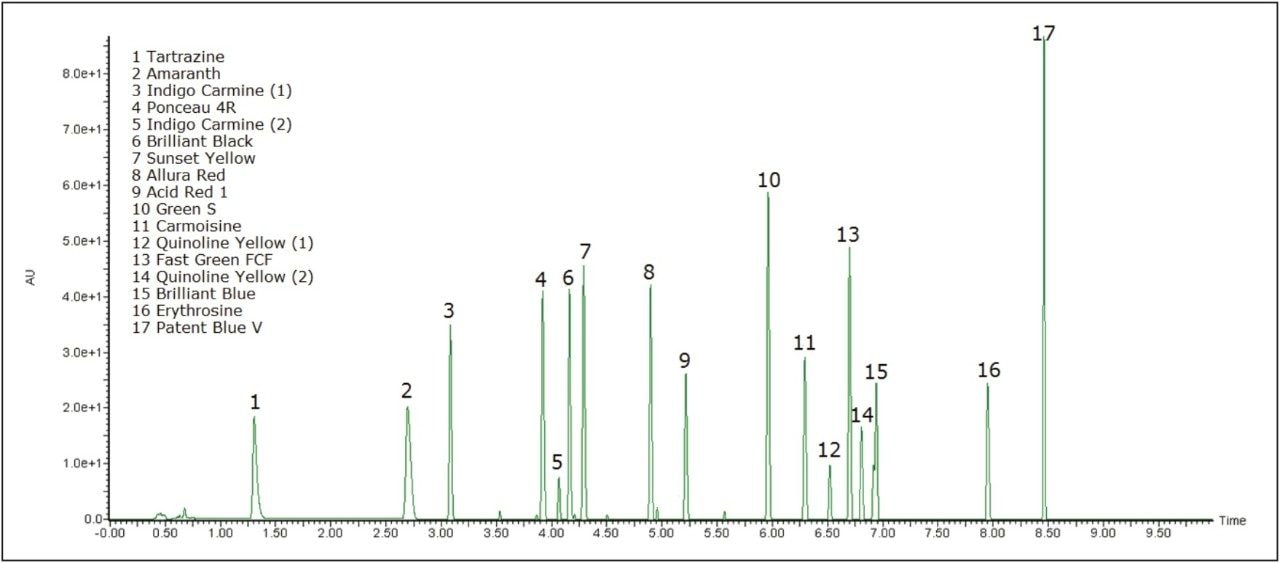
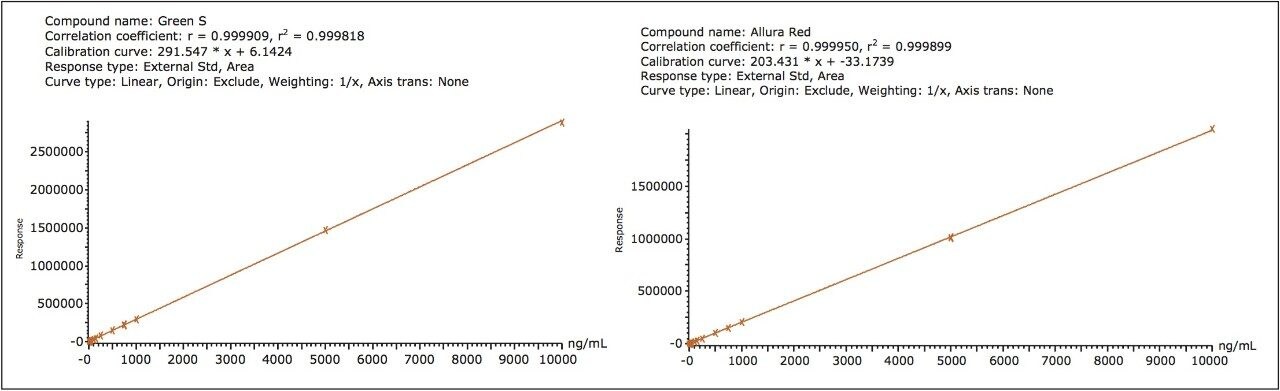
Figure 1 shows the 15 dyes and selected isomers that were separated with the ACQUITY UPLC H-Class System and PDA eλ Detector. Excellent linearity was achieved for all of the dyes with ranges spanning up to 4.5 orders of magnitude and many with R2 values >0.999. Figure 2 shows examples of these calibration curves with Green S and Allura Red.
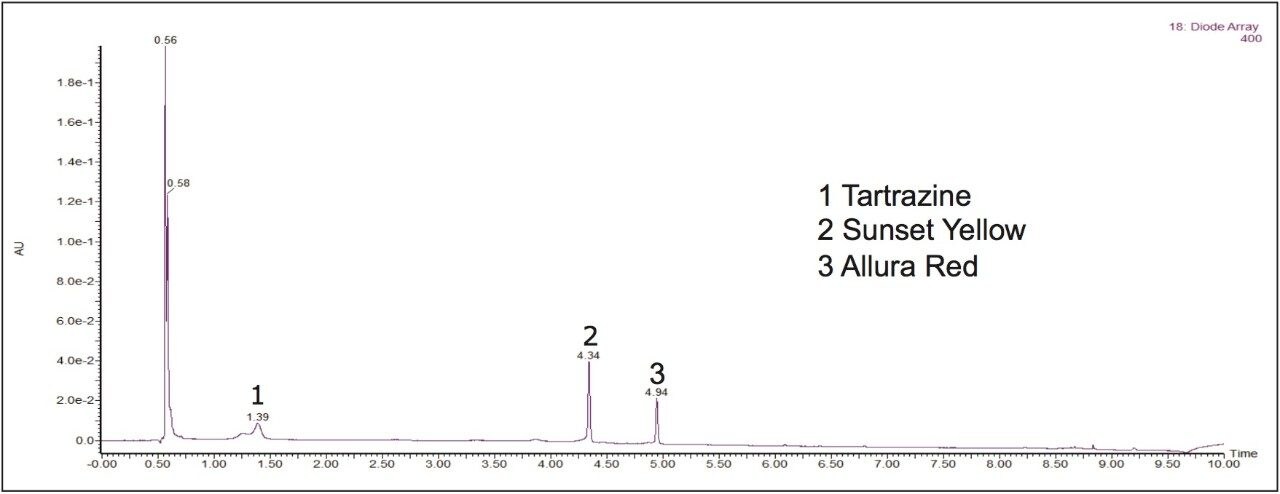
An identification example of artificial food dyes in a dilution of a lemon-flavored iced tea powdered drink mix sample is shown in Figure 3. The PDA eλ Detector provided the sensitivity and selectivity necessary to accurately characterize the synthetic color additives present in the sample.
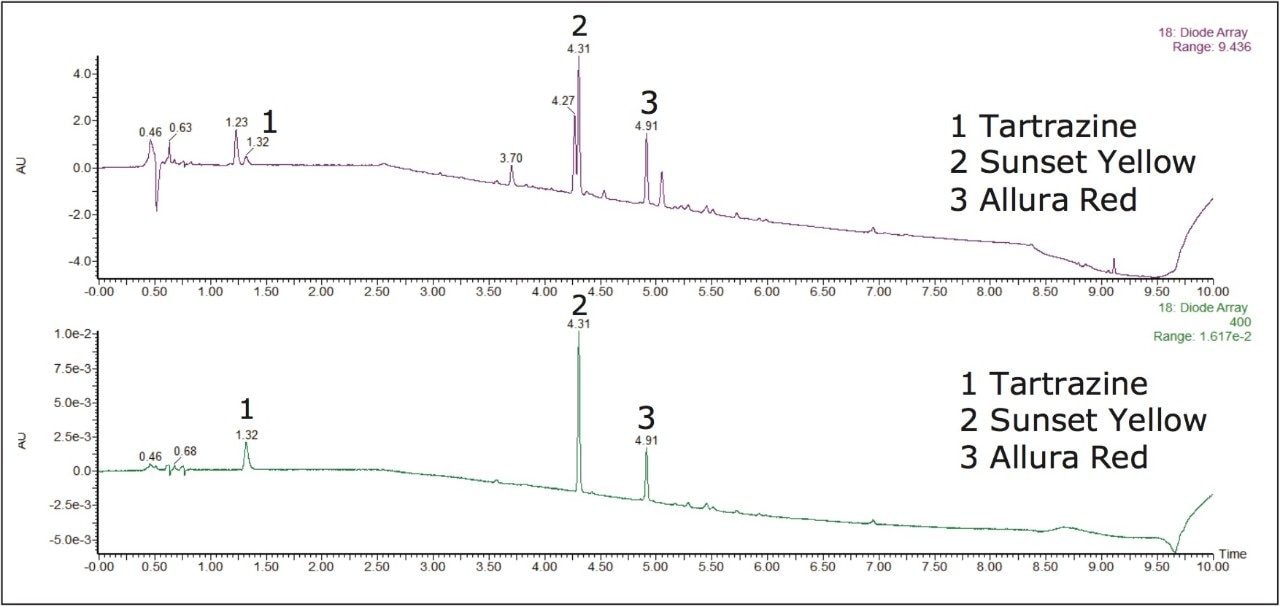
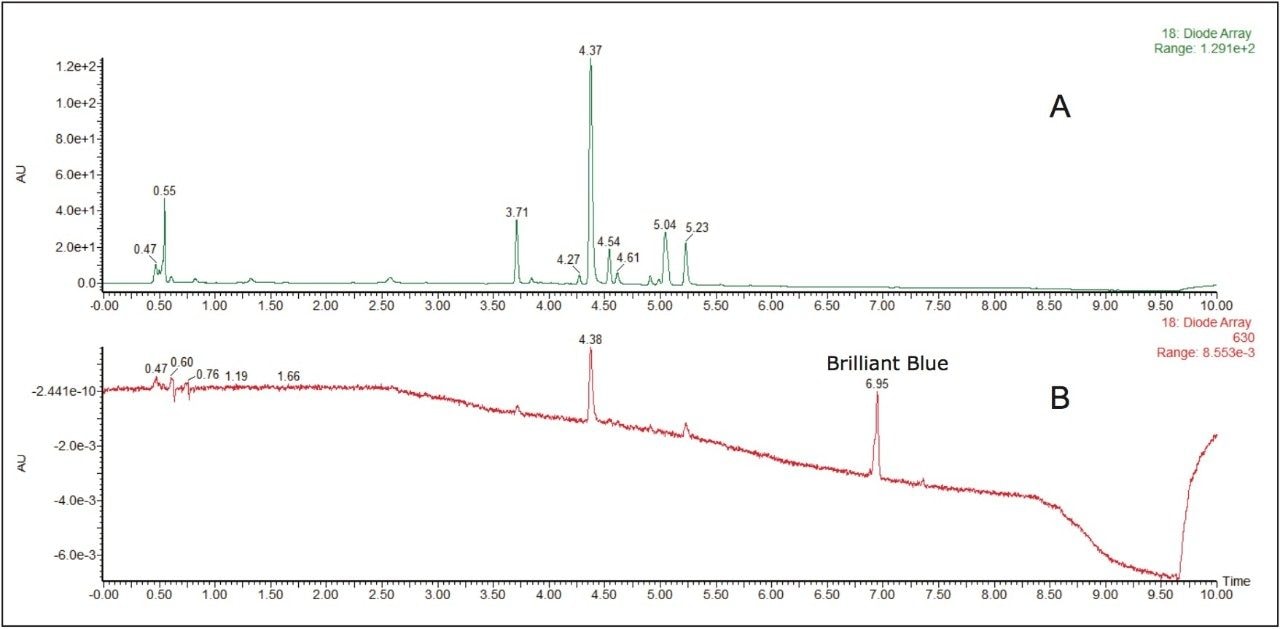
Tartrazine, Sunset Yellow, and Allura Red were identified in the iced tea drink mix. While matrix interferences existed in chromatogram A, extracting a wavelength within the visible region, shown in chromatogram B, can eliminate those interferences and allow for a straightforward quantitative analysis of these compounds. With the extended wavelength PDA, wavelengths from 190 nm to 800 nm can be analyzed potentially providing advantages for the analysis of yellow and blue dyes. Figure 4 shows how Brilliant Blue in a peach mango drink mix sample was easily identifiable at 6.95 minutes when a chromatogram at 630 nm was extracted with the PDA.
The analysis of artificial food dyes with ACQUITY UPLC H-Class System and the ACQUITY UPLC PDA eλ Detector is also useful for even complicated matrices. Figure 5 shows the identification of Tartrazine, Sunset Yellow, and Allura Red in a smoked salmon sample with this method.
To assess the performance of the method in a typical matrix, 100 ng/mL of Carmoisine was spiked into a sample of an orange hard candy. The results are presented in Table 1.
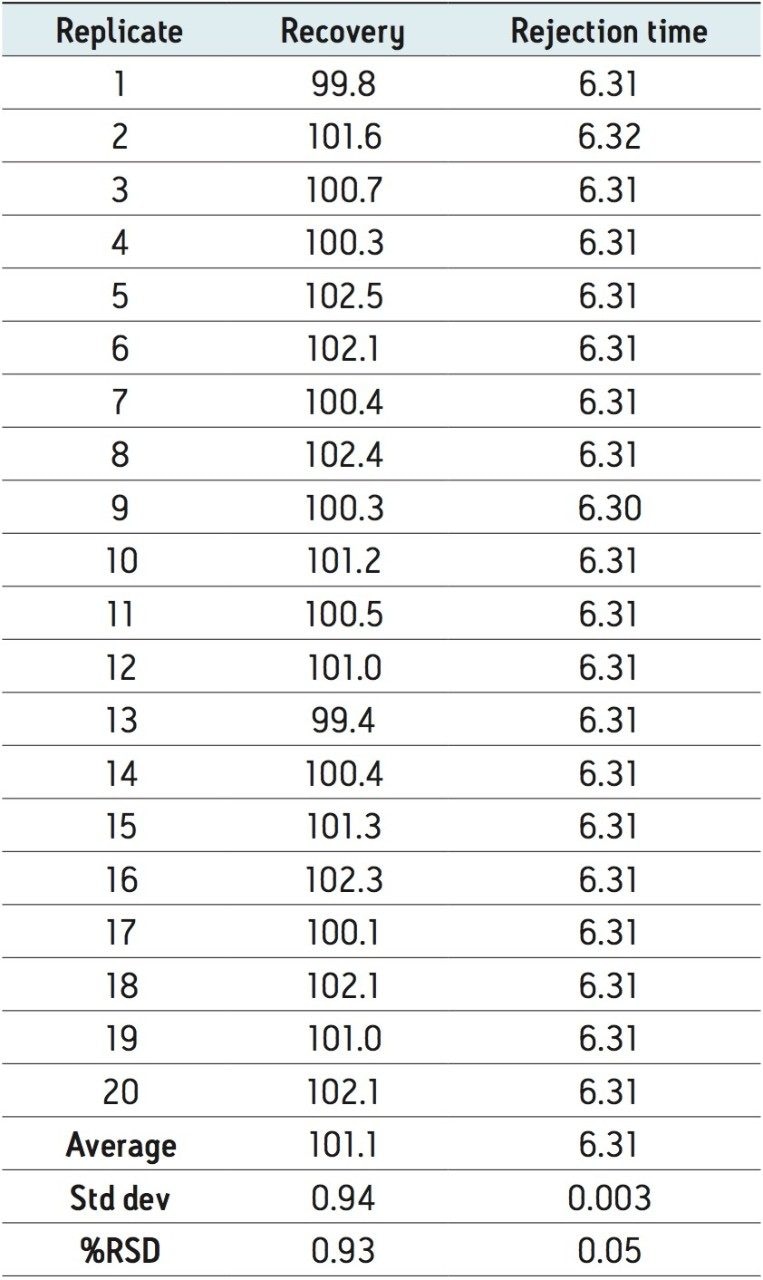
Excellent repeatability was observed for the retention time of Carmoisine over 20 injections with a relative standard deviation (RSD) of 0.05%. An average recovery of 101.1% was also determined.
An efficient and accurate method has been developed for the analysis of artificial food dyes in foods and beverages. This method offers a rapid, quantitative analysis of synthetic color additives in a shorter timeframe compared to traditional methods. By extracting wavelengths in the visible region, excellent selectivity and sensitivity can be achieved even when analyzing more complex matrices.
720005217, October 2014