
The Ability to successfully run compendia methods on an ACQUITY UPLC H-Class System is described utilizing high percentage organic solvent as diluent without peak distortion.
To investigate the means for a simple solution to overcome the effect of strong solvents in the sample diluent, five United States Pharmacopeia (USP) methods were selected (i.e. acetaminophen, itraconazole, ketoconazole, loratidine, and bicalutamide) which require sample diluent organic levels ranging between 67%–100% organic.
All methods were conducted on the ACQUITY UPLC H-Class System with dispersion volumes calculated for every configuration used. These were combined with structured and iterative modifications to increase pre-column volume to assess the impact of additional pre-column volume on peak symmetry problems brought on as a result of high organic diluents.
Ability to successfully run compendial methods on an ACQUITY UPLC H-Class System utilizing high percentage organic solvent as diluent without peak distortion.
Ideally when running a chromatographic method, the sample diluent composition should be as close to the method starting conditions as possible. This is done in order to minimize the possibility of band spreading and peak distortion from sample solvent effects, which can lead to poor peak symmetry, peak splitting, or unusable data.
The cause for these effects is a difference in elutropic strength between the diluent and the mobile phase. Peak broadening and shape abnormalities generally get worse as the diluent becomes stronger than the mobile phase.1-2
In fact, it is widely recognized that the sample injected should ideally be dissolved in the starting mobile phase conditions. However the pre-treatment of a given sample often ends in the analytes dissolved in a solvent composition very different from that used in the mobile phase. In order to prevent solubility and poor peak shape problems, many protocols require evaporation of the sample solvent in pre-treatment and reconstitution in mobile phase. However, this added step is a time consuming process that often takes longer than the HPLC analysis.1
The practice widely recommended is to avoid stronger solvents than the mobile phase to dissolve samples and standards. The underlying assumption is that an injection solvent stronger than the mobile phase can interfere with the adsorption of the sample at the column head, especially when large injection volumes are used.2 Unfortunately, in practice this is not always possible as sample solubility often dictates the amount of organic content needed to ensure complete dissolution.
With older, higher dispersion volume LC systems, this phenomenon is less problematic due to sufficient pre-column sample/solvent/mobile phase mixing which mitigated peak problems brought about by solvent effects.
However, for modern lower dispersion UHPLC systems, high organic diluents can be problematic when injected in larger volumes and can result in poor peak symmetry or splitting.
To understand this phenomenon and investigate the means for a simple solution to overcome the effect of strong solvents in the sample diluent, five United States Pharmacopeia (USP) methods were selected (i.e. acetaminophen, itraconazole, ketoconazole, loratidine, and bicalutamide) which require sample diluent organic levels ranging between 67%–100% organic.
All methods were conducted on the ACQUITY UPLC H-Class System with dispersion volumes calculated for every configuration used. These were combined with structured and iterative modifications to increase pre-column volume to assess the impact of additional pre-column volume on peak symmetry problems brought on as a result of high organic diluents.
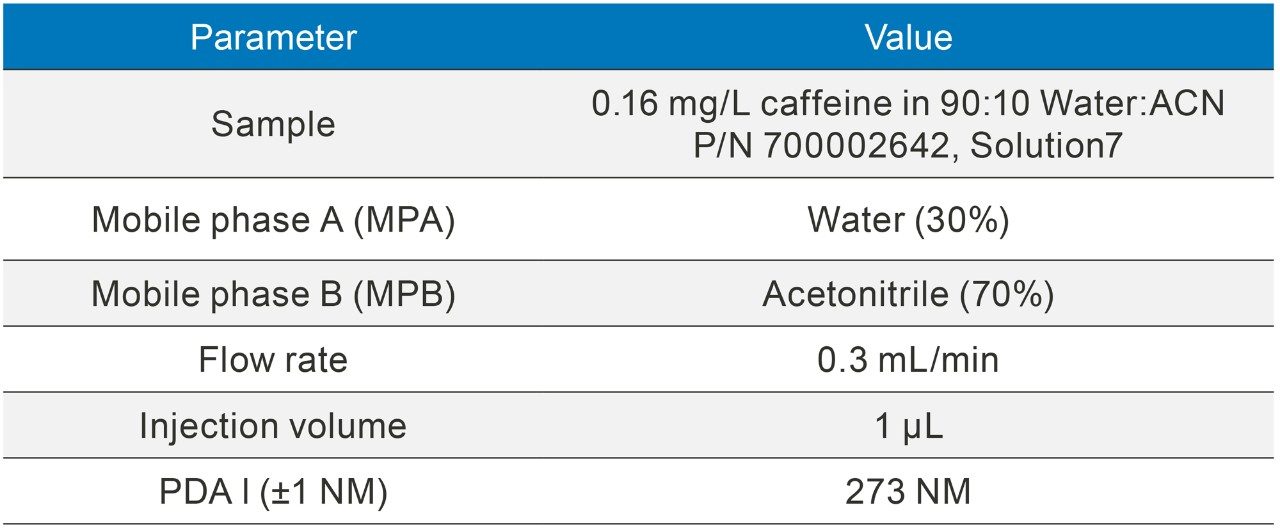
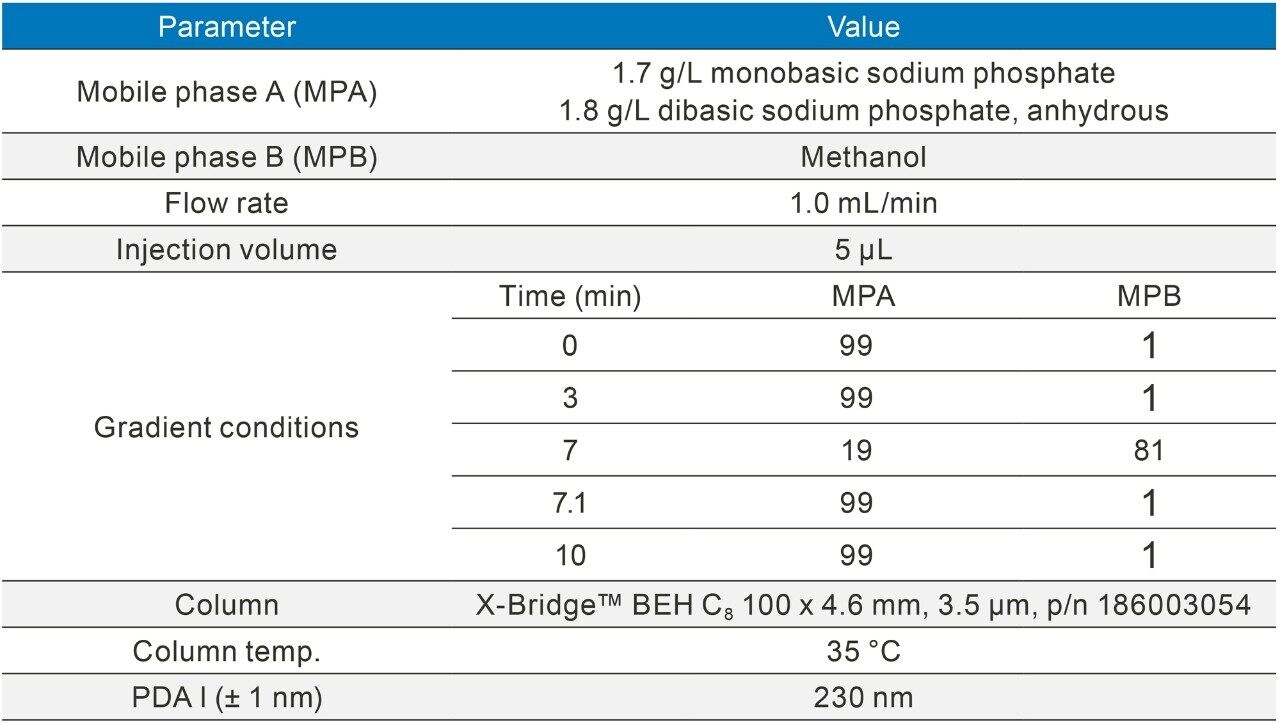
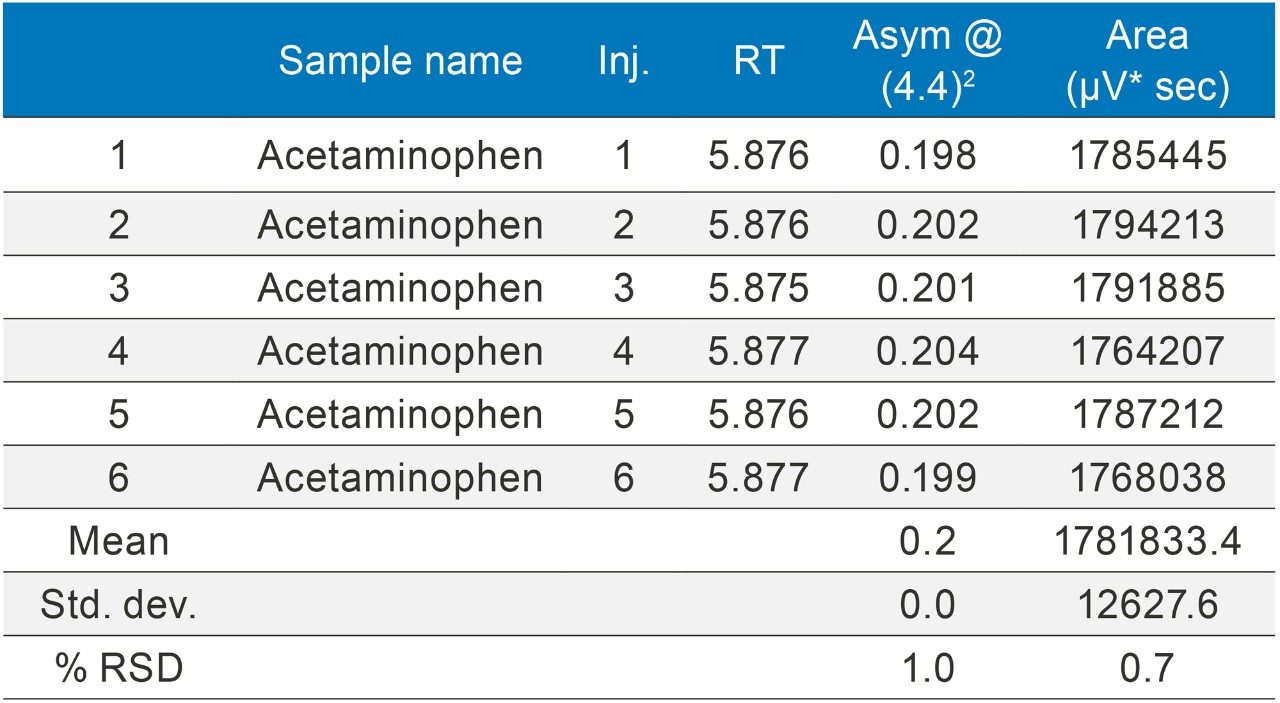
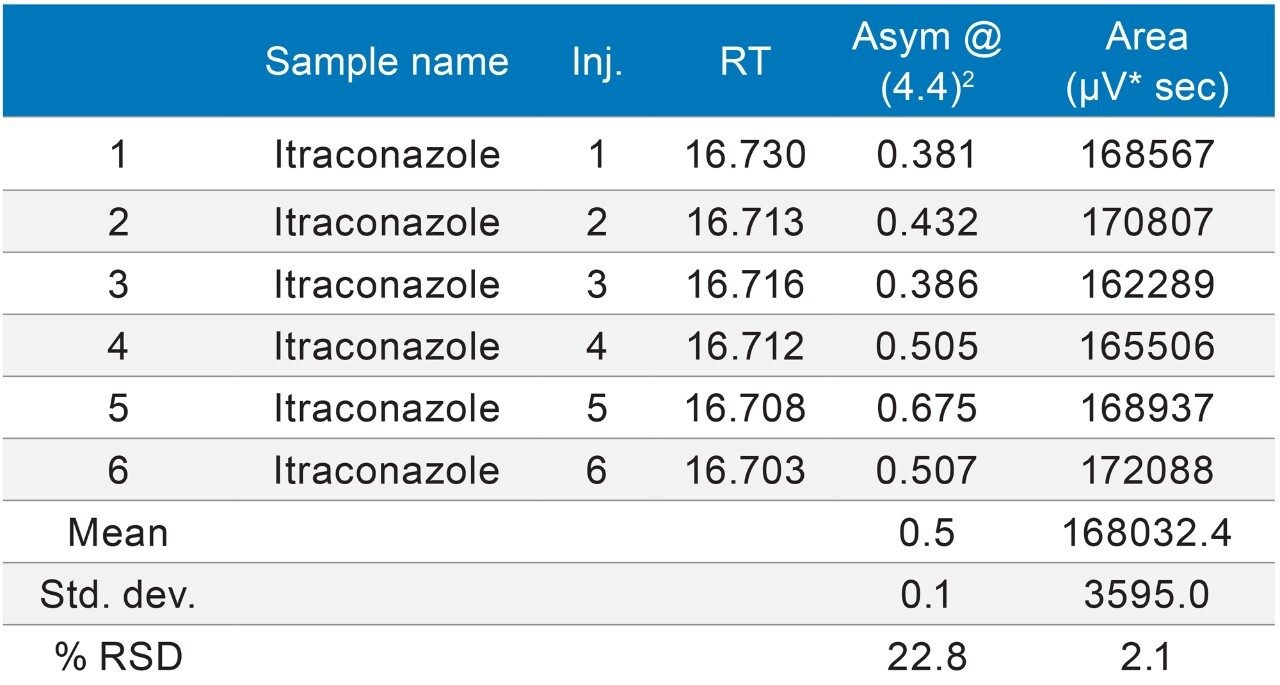
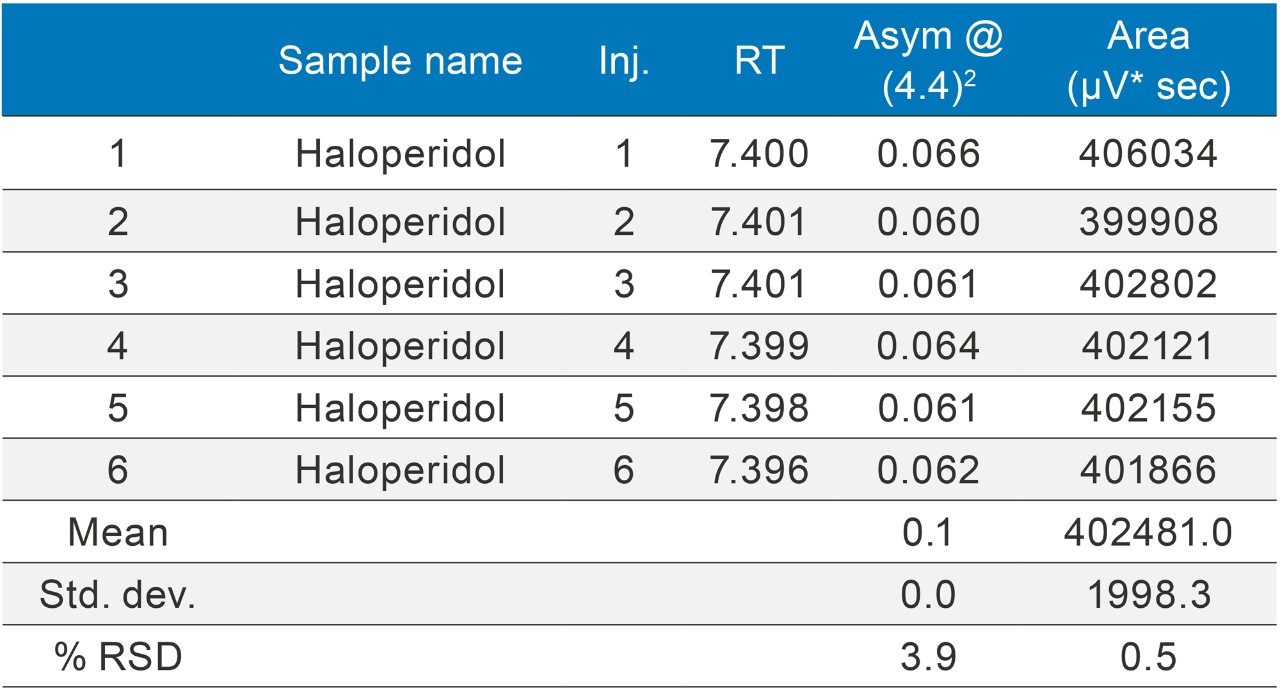
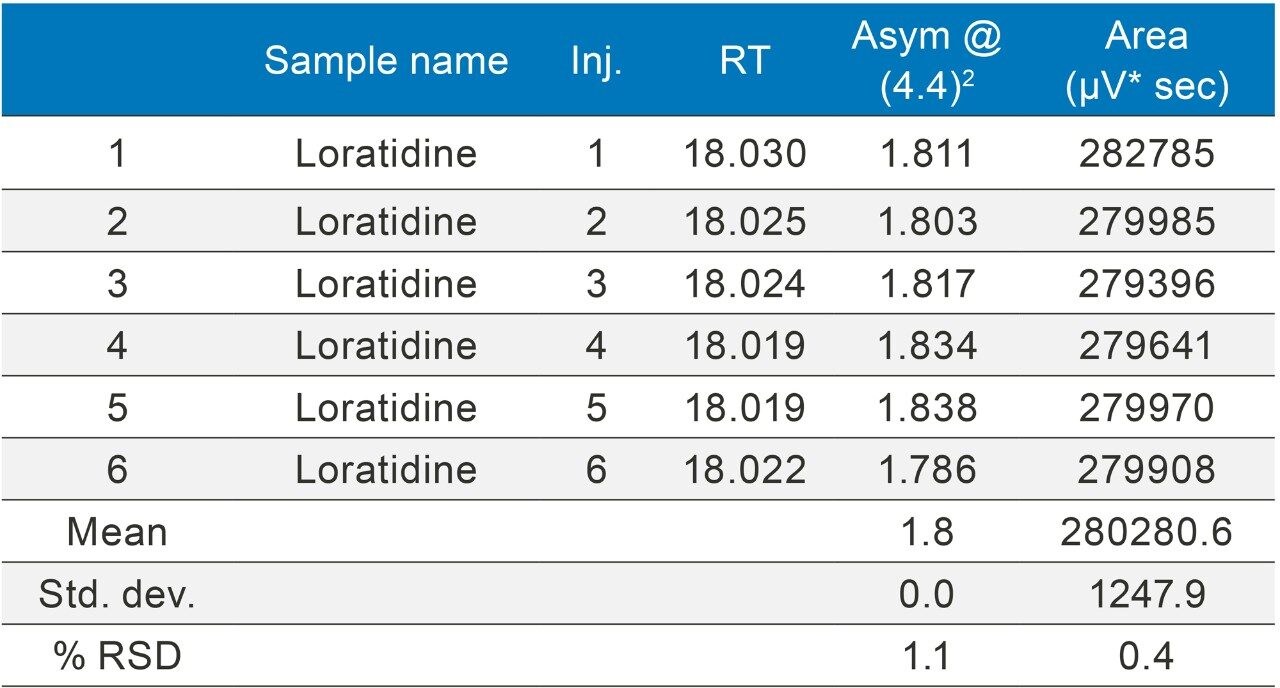
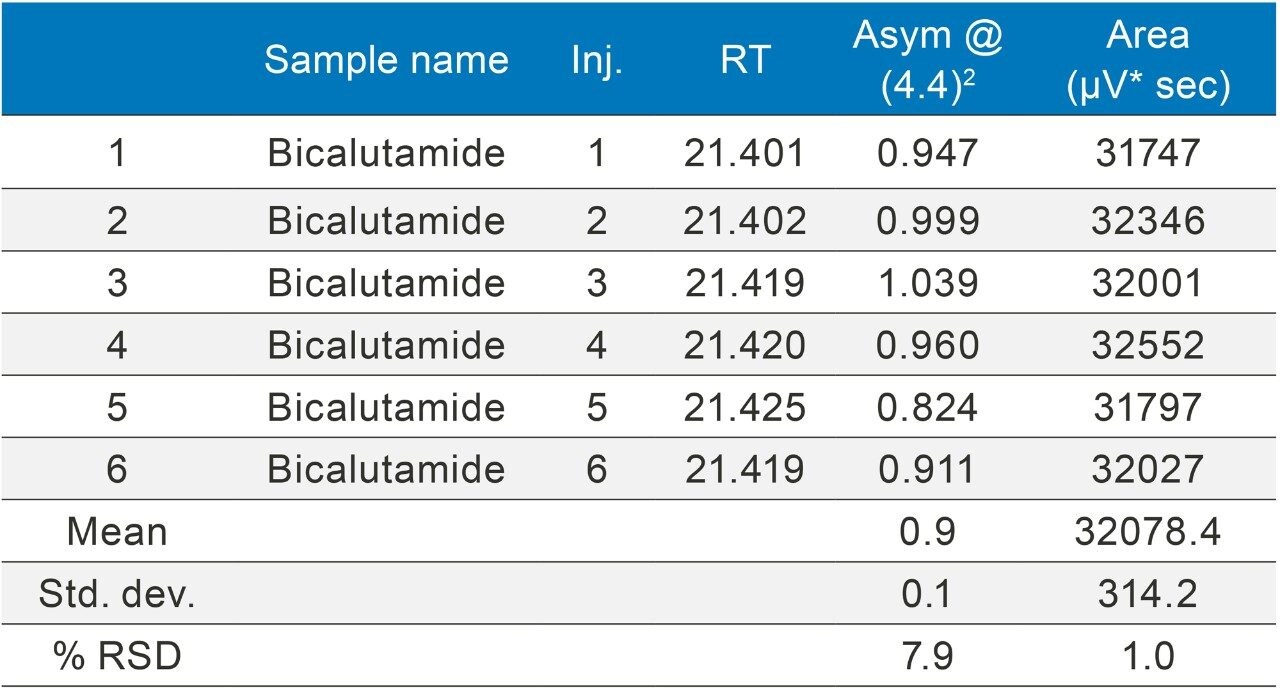
The methods were selected from the USP and the standards were analysed six times (n=6). The standards were prepared as per USP methods (Table 1).
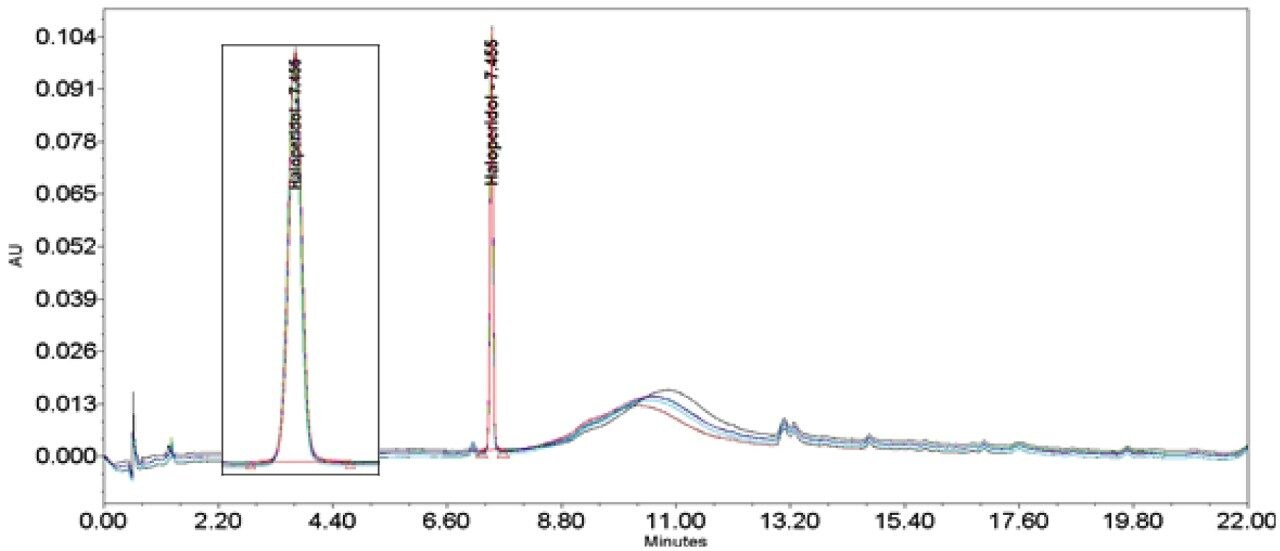
The methods were run on an ACQUITY UPLC H-Class System with and without a 50 µL loop inserted between position six of the injector pod located in the sample manager and the column inlet tubing as shown in (Figure 1). Under both conditions, the chromatography was appraised visually, the mean of the peak asymmetry at 4.4% peak height and the % RSD of the peak area were compared as indicators of chromatographic performance in this study.
All methods with the exception of loratidine utilized the column manager (CM-A). Loratidine, required the CH30-A ACQUITY Column Heater to accommodate the 25 cm HPLC column detailed in the USP method.

The materials used for analysis include (analytical standards in bold):
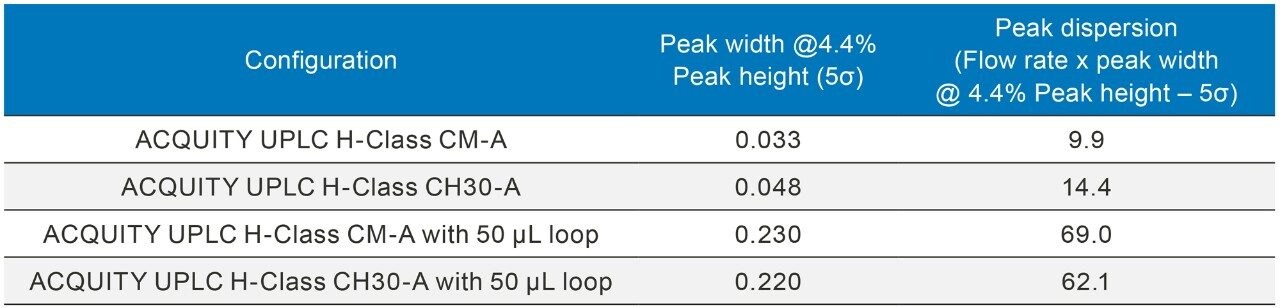
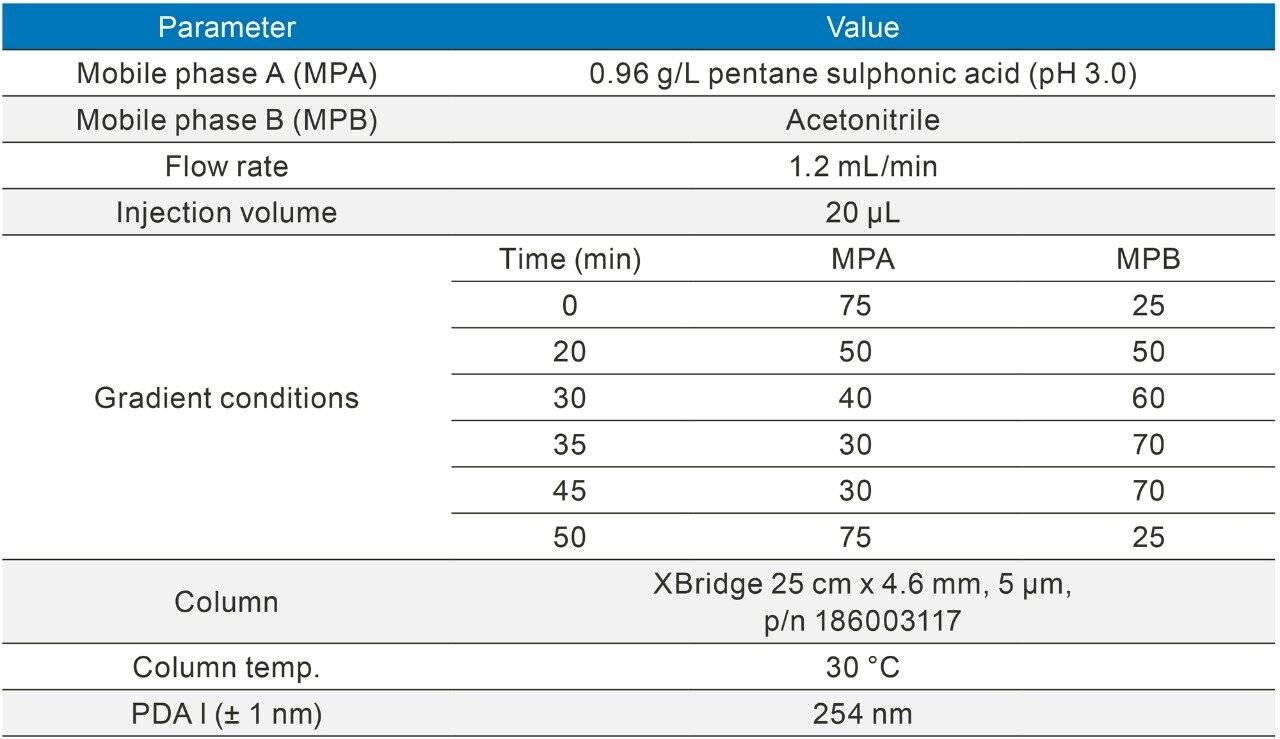
Prior to analysis, the dispersion volume for all configurations were calculated using a blank union (P/N 700002636) under the following conditions (Table 2).
Extra column dispersion (μL) was calculated as the peak width (minutes) at 4.4% peak height (5σ) multiplied by the flow rate (μL per minute).

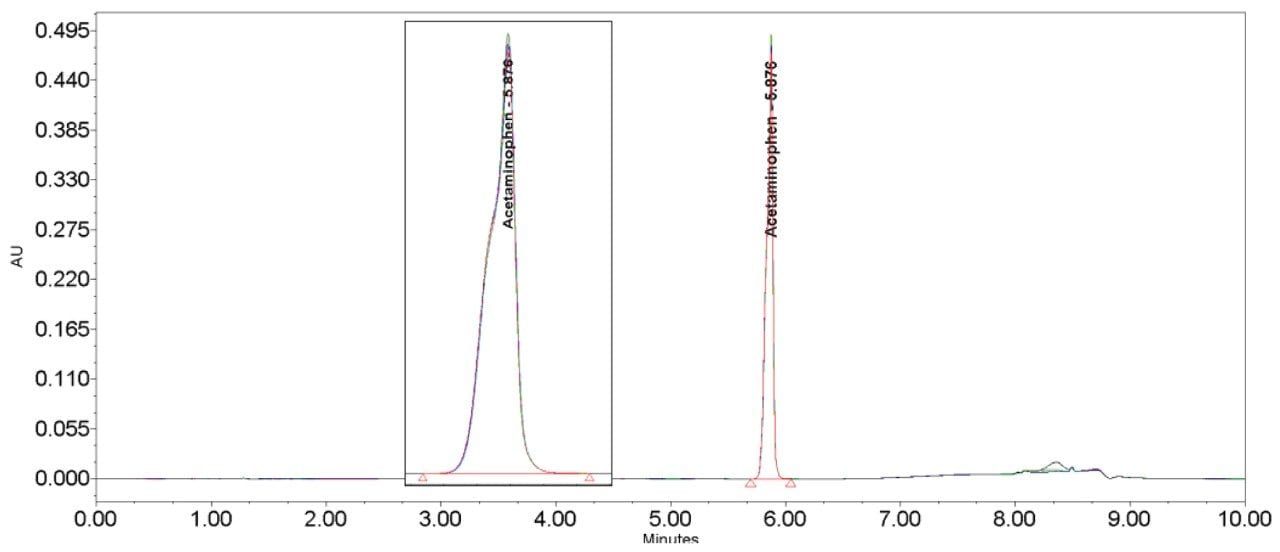
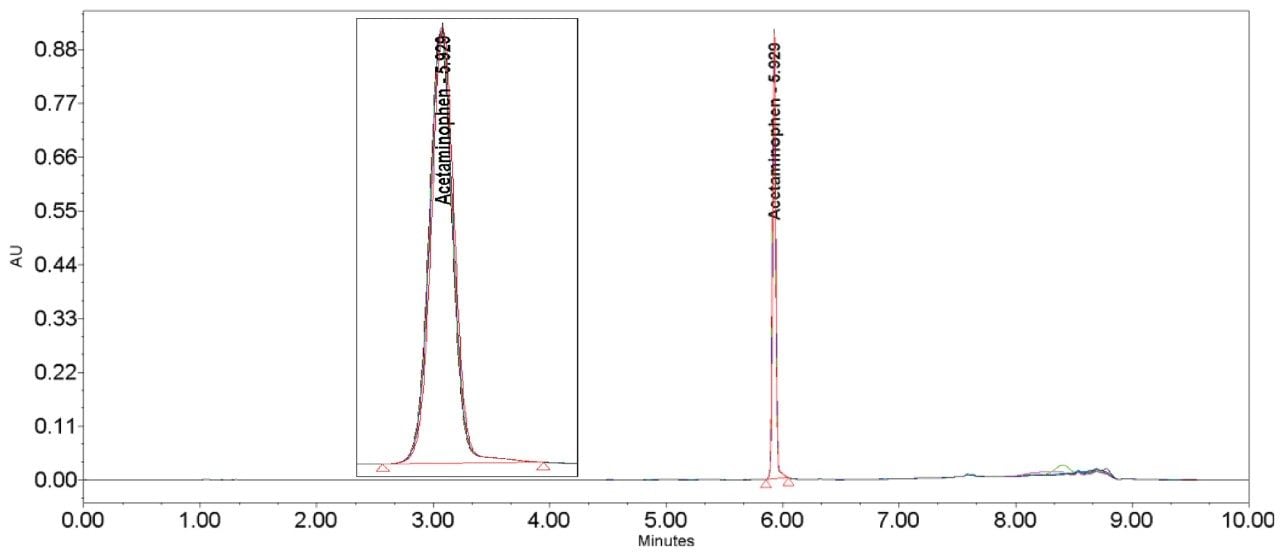
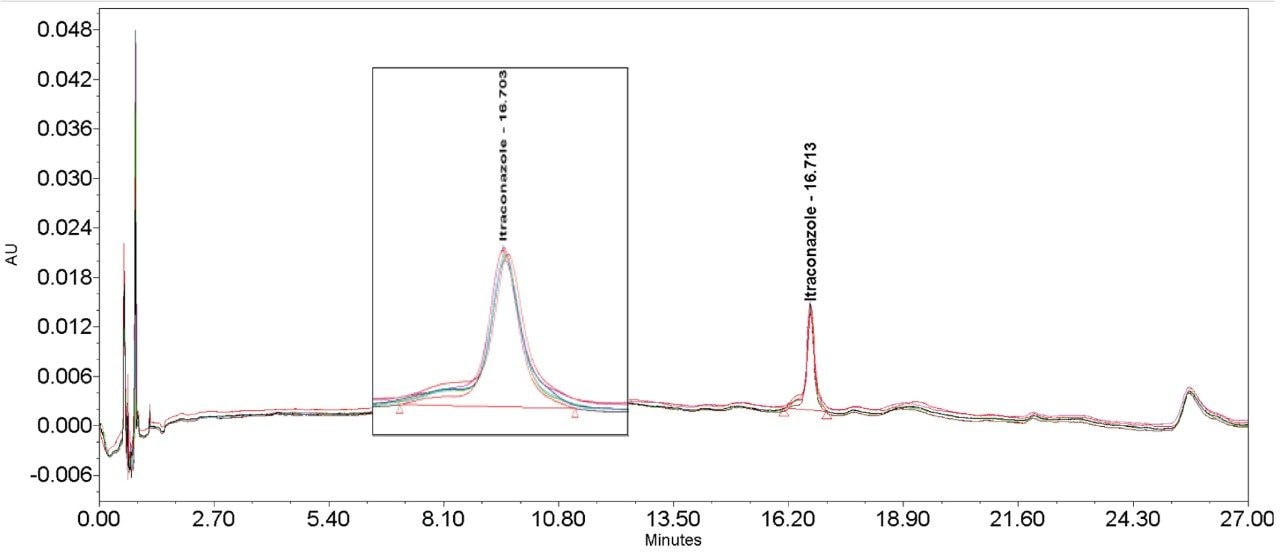
The inclusion of additional pre-column volume, i.e. a 50 μL loop on the ACQUITY UPLC H-Class System, has significantly improved the peak shape of acetaminophen (Figures 3–4/Tables 5–6), itraconazole (Figures 5–6/Tables 8–9), haloperidol (Figures 7–8/Tables 11–12) and loratidine (Figures 9–10/Tables 15–16). Peak symmetry improved noticeably for acetaminophen (0.2 to 1.0) and haloperidol (0.1 to 1.0). These compounds also exhibited an improvement in peak area % RSD (Table 22).


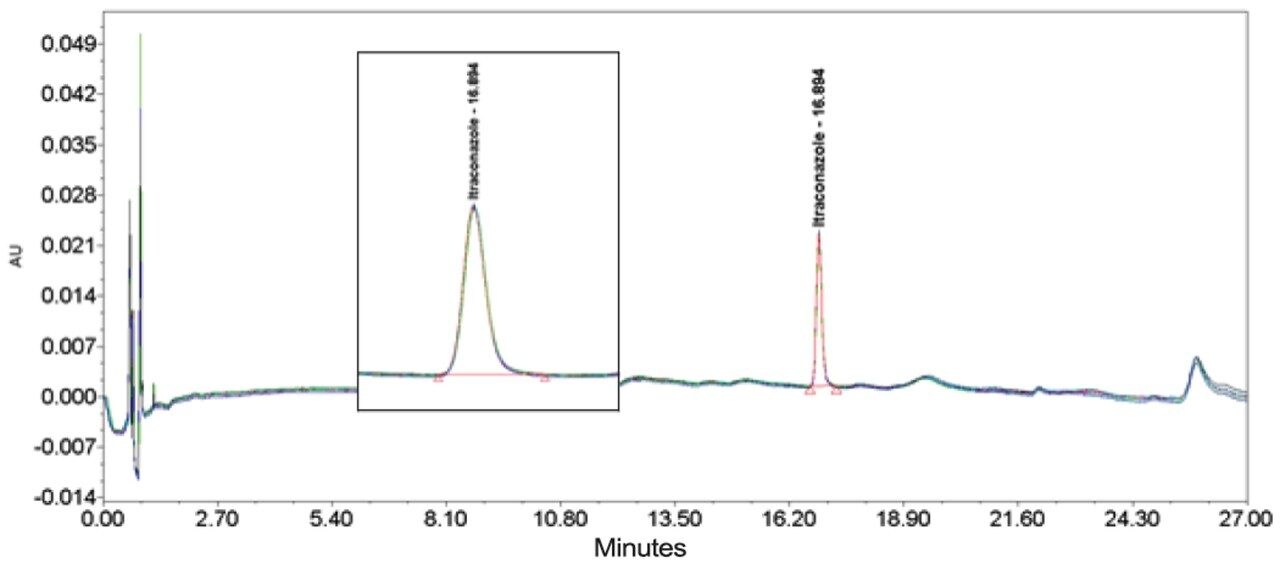




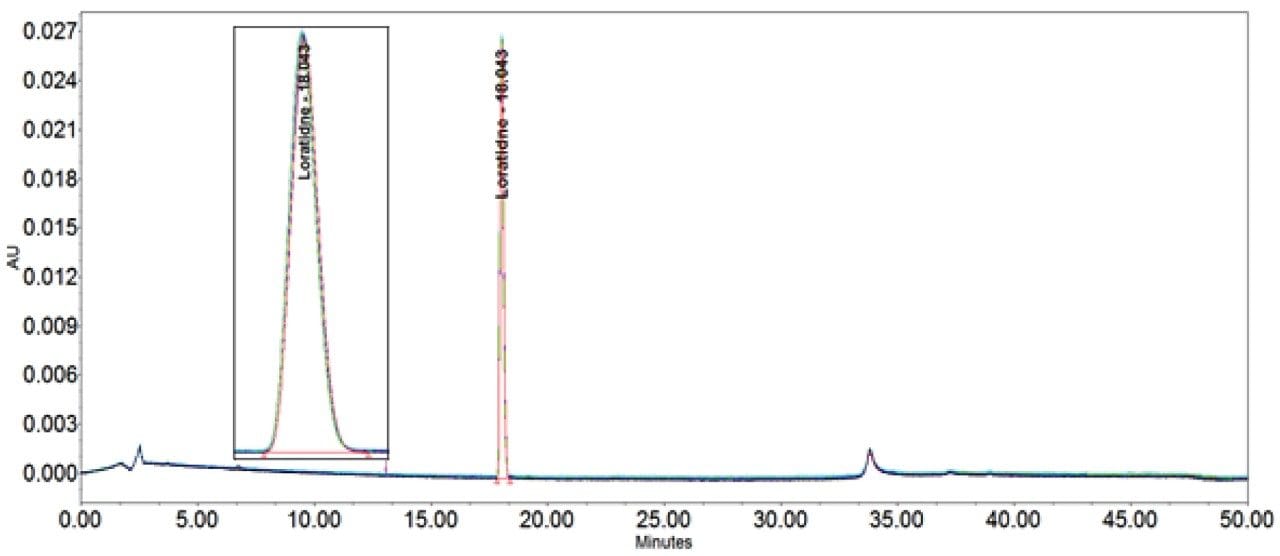

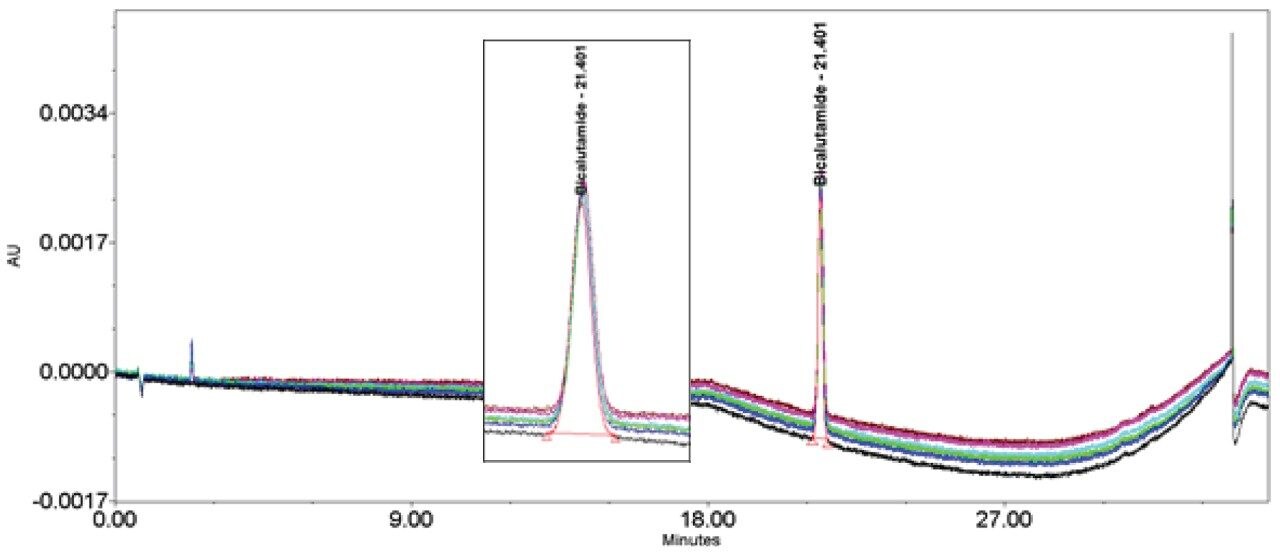
Bicalutamide (Figures 11–12/Tables 17–18) showed no significant peak distortion without the loop and no significant impact (positive or negative) with the use of the additional pre-column volume. This may be due to the relatively low concentration of organic diluent (i.e. 67%).

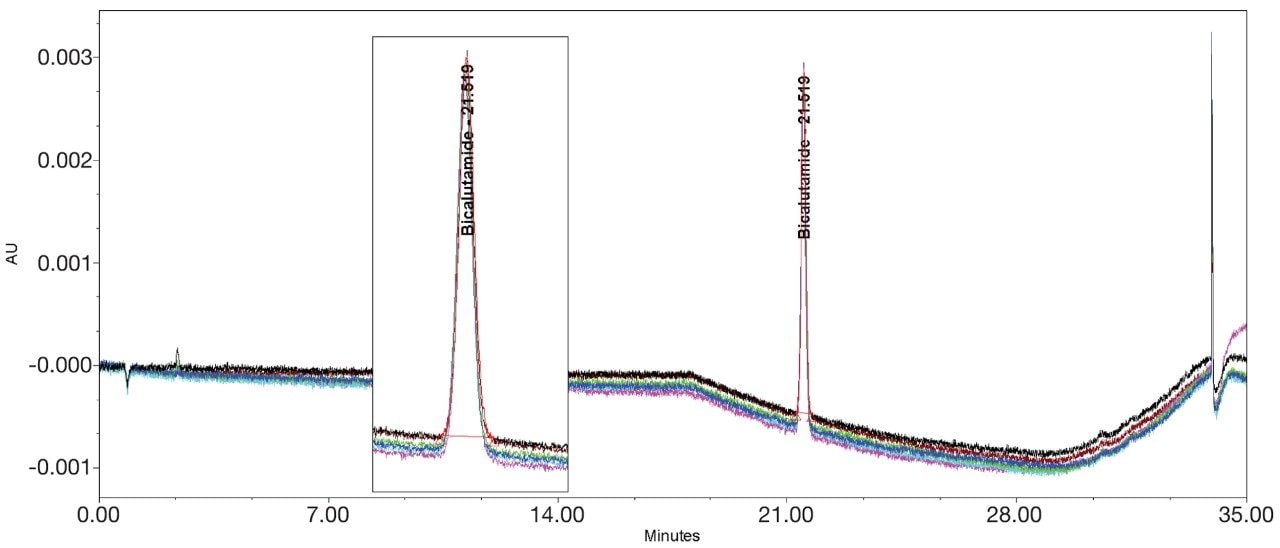


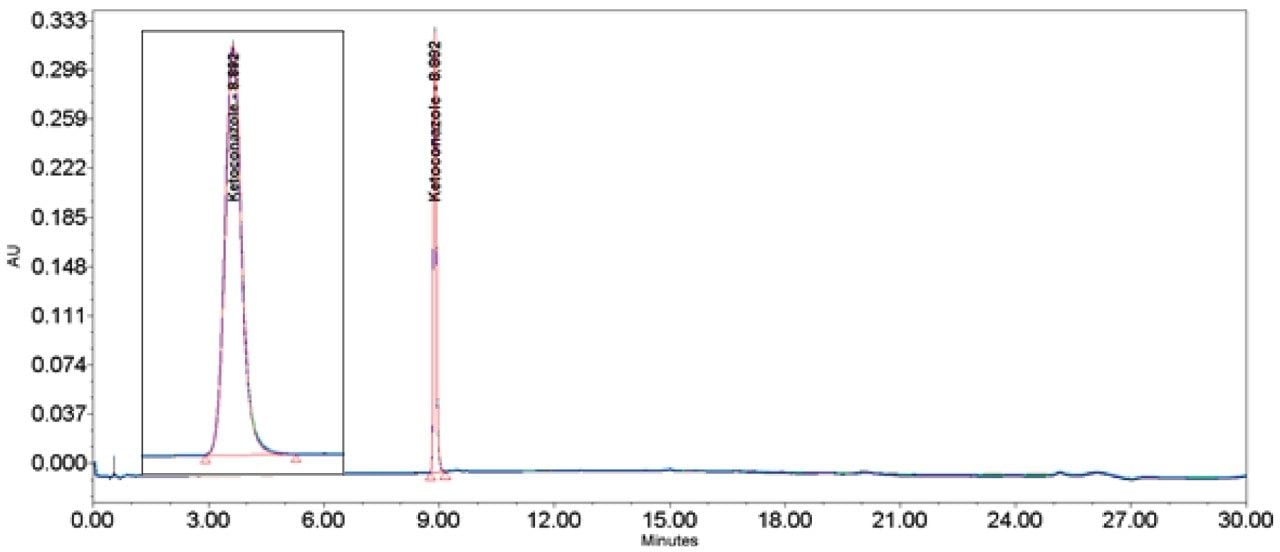
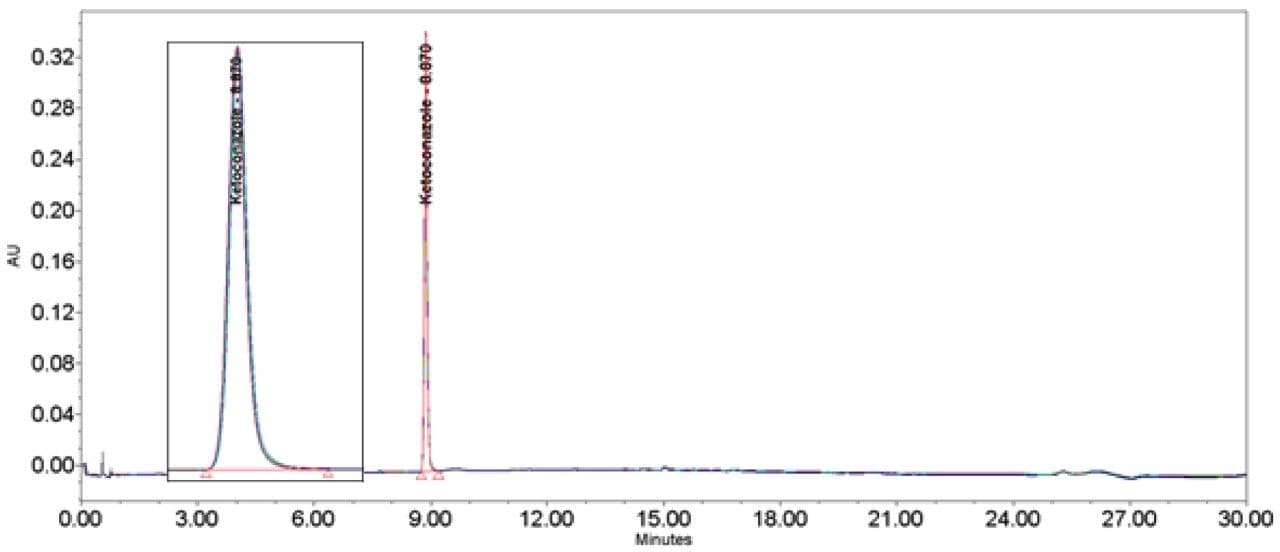
Ketoconazole (Figures 13–14/Tables 20–21) also showed no significant peak distortion without the loop and no significant impact (positive or negative) w ith the use of the additional pre-column volume despite having 100% methanol as diluent. This may be explained by the presence of organic component in both Mobile phase A and B, facilitating sufficient mixing prior to injection onto the column. Further work would be required to verify this theory.
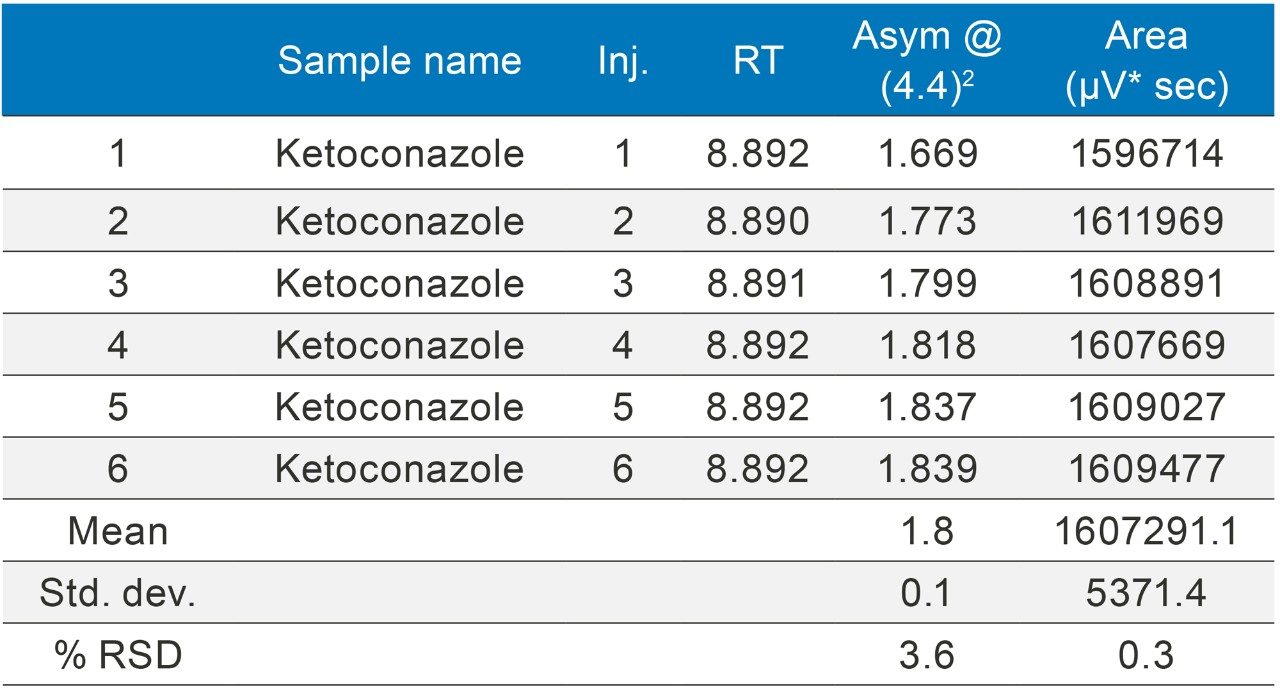

All results are summarized in Table 22.
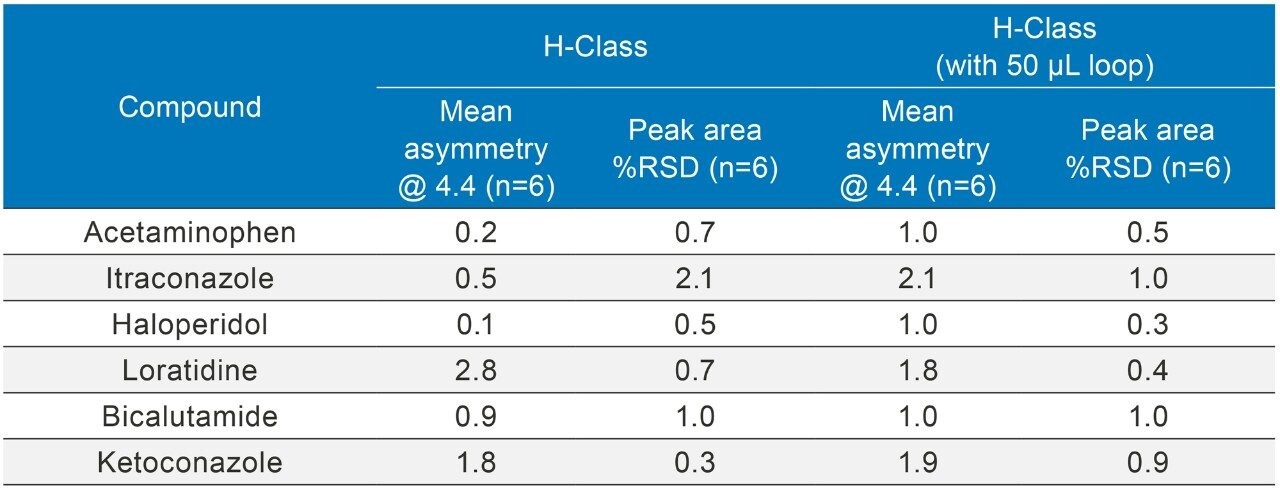
The addition of pre-column volume by utilizing a 50 µL loop has had a significant impact on the peak shape and peak area %RSD for acetaminophen, itraconazole, haloperidol, and loratidine indicating that it is a valid strategy for overcoming peak distortions when working with high organic diluent on a low dispersion LC system.
This solution would enable customers bound by compendial method parameters to overcome unsatisfactory/non-usable data with a simple fix that would be fully supported in a regulatory compliant laboratory environment.
720006242, March 2018