Fully Automated Solid Phase Extraction Sample Preparation, using the Andrew+™ Pipetting Robot configured with the Extraction+ Connected Device
Abstract
The following work demonstrates the new capabilities and flexibility of the Andrew+ Pipetting Robot in combination with the Extraction+ Connected Device, controlled by OneLab™ Software, for fully automated Solid Phase Extraction (SPE) sample preparation in cartridge and plate SPE formats.
Benefits
- Fully automated SPE sample extraction using the Andrew+ Pipetting Robot and the Extraction+ Connected Device, eliminating the need for user intervention
- Automation compatibility with SPE plates and cartridge formats
- Liquid transfer flexibility into collection in plates, tubes, and bottles
- Fully programmable vacuum pressure profiles extraction performance variability
- Automated sample preparation and extraction increase efficiency, providing walk-away time for the analyst
- Transferrable methods on the easy-to-use OneLab Software allow for easy implementation of the same preparation procedure across users and labs
Introduction
Liquid chromatography-mass spectrometry (LC-MS) bioanalytical SPE sample preparation is time-consuming and complex, often perceived as the “bottle-neck” in the LC-MS bioanalytical workflow, due to the many protocol steps required to accurately and reliably isolate or purify the target analyte(s) from various biological matrices. Implementing fully automated SPE sample preparation greatly simplifies the analytical workflow, reduces human error, and improves analytical method reproducibility (day-to-day, user-to-user, and lab-to-lab), while freeing up the analyst to do other tasks.
The work presented herein demonstrates fully automated sample preparation and SPE extraction solution using the Andrew+ Pipetting Robot (Andrew+) and newly introduced Extraction+ Connected Device (Extraction+), controlled via OneLab Software (Figure 1), to ensure accurate, reliable, and robust SPE sample preparation.

Experimental
LC-MS Conditions
|
LC system: |
ACQUITY UPLC I-Class, FL with Column Manager (CMA) |
|
MPA: |
0.1% FA in Water |
|
MPB: |
0.1% FA in Acetonitrile |
|
Column/Sorbents: |
HSS PFP 1.8 µm, 2.1 mm x 50 mm Column (p/n: 186005965) |
|
Column temp.: |
35 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
5 µL |
|
WNW: |
9:10 water:acetonitrile |
|
SNW: |
25:25:25:25 water:methanol:acetonitrile:water |
|
MS system: |
Xevo® TQ-XS |
|
Capillary (Kv): |
3.0 |
|
Cone voltage: |
30 V |
|
Desolvation temp.: |
500 °C |
|
Desolvation flow: |
1100 L/Hr |
|
Cone gas flow: |
150 L/Hr |
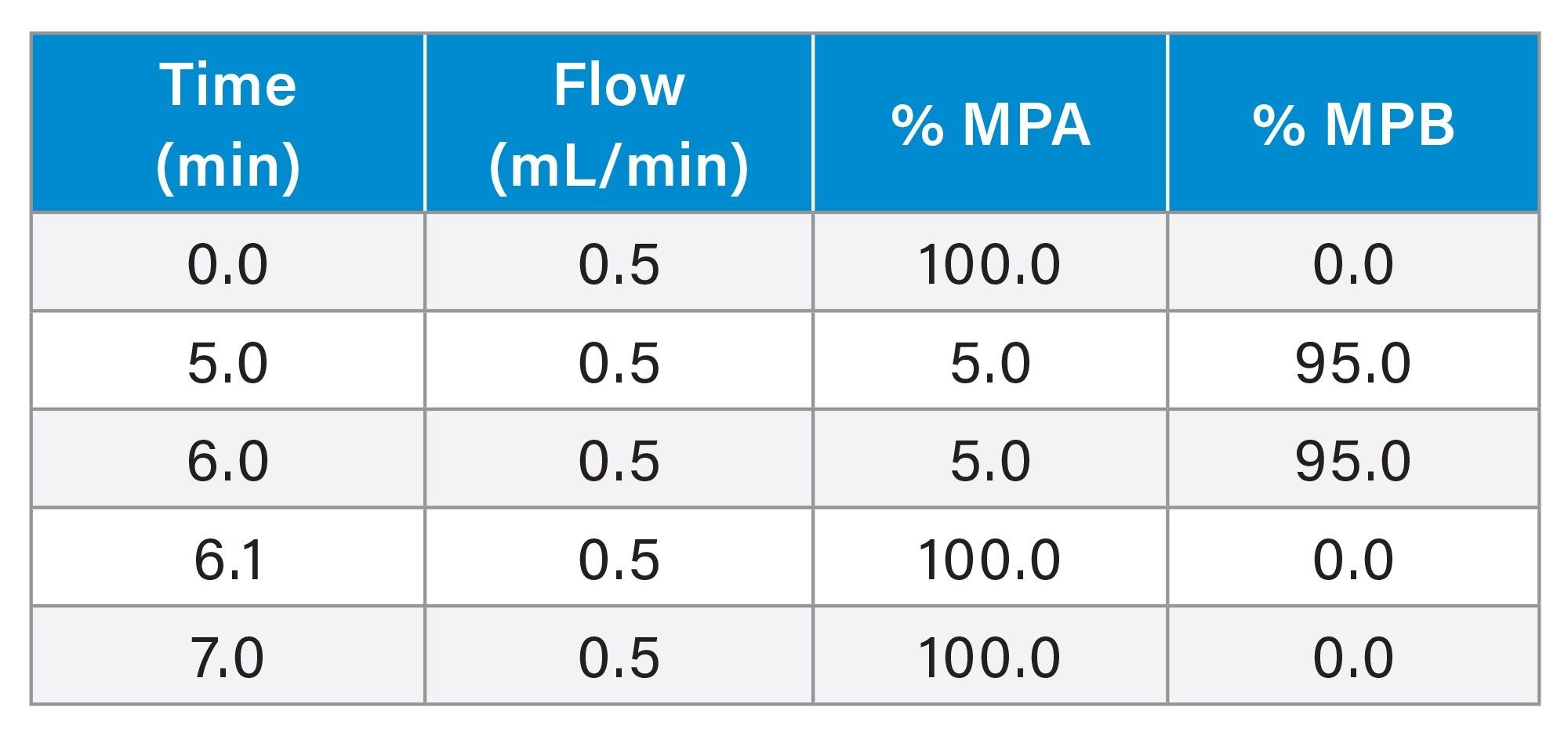
LC Gradient
MS Settings
|
Ionization mode: |
ESI+ |
|
Acquisition range: |
MRM |
|
Capillary voltage: |
2.00 kV |
|
Cone voltage: |
60 V |
|
Desolvation temperature: |
500 °C |
|
Desolvation flow: |
1000 L/Hr |
|
Cone gas flow: |
150 L/Hr |
|
Collision gas flow: |
0.2 mL/min |
|
Nebulizer gas flow: |
7 Bar |
Data Management
|
Instrument control software: |
MassLynx™ (v4.2) |
|
Quantification software: |
TargetLynx™ |
Sample Preparation and SPE Extraction
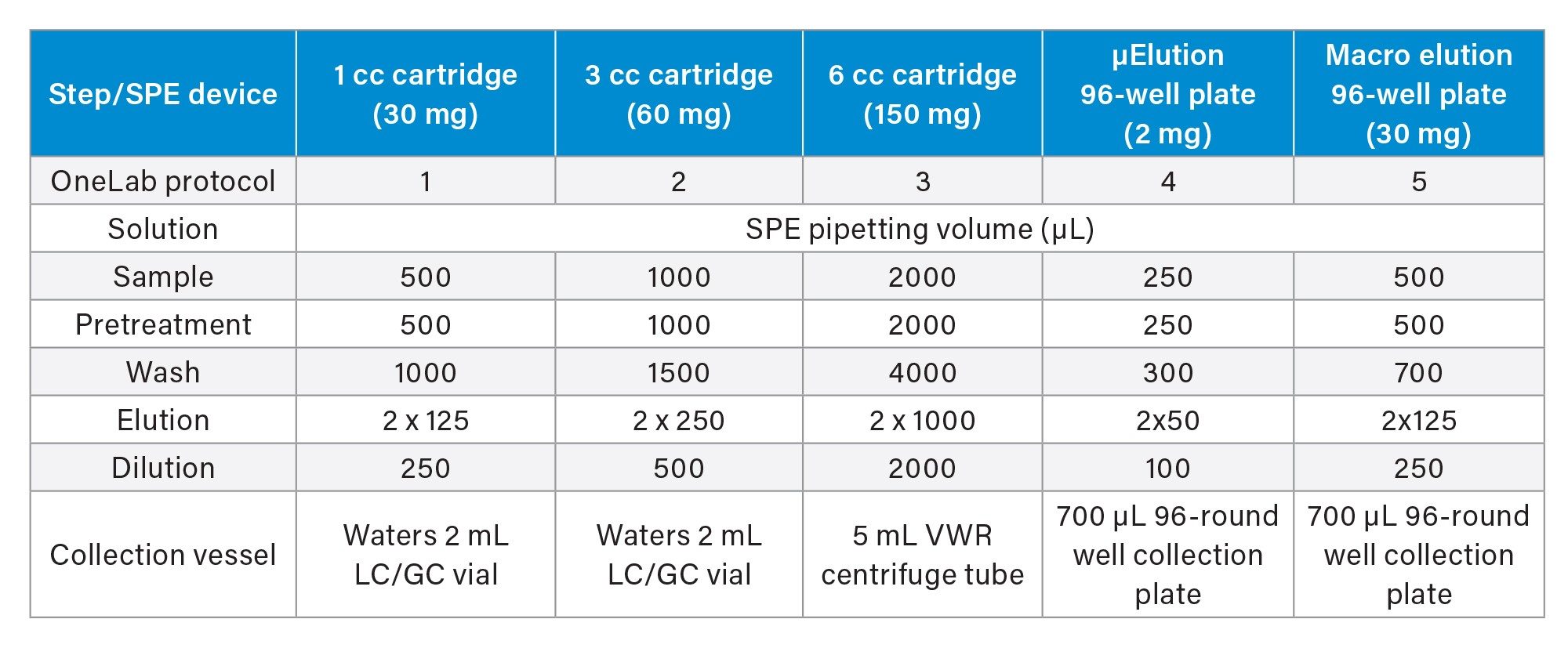
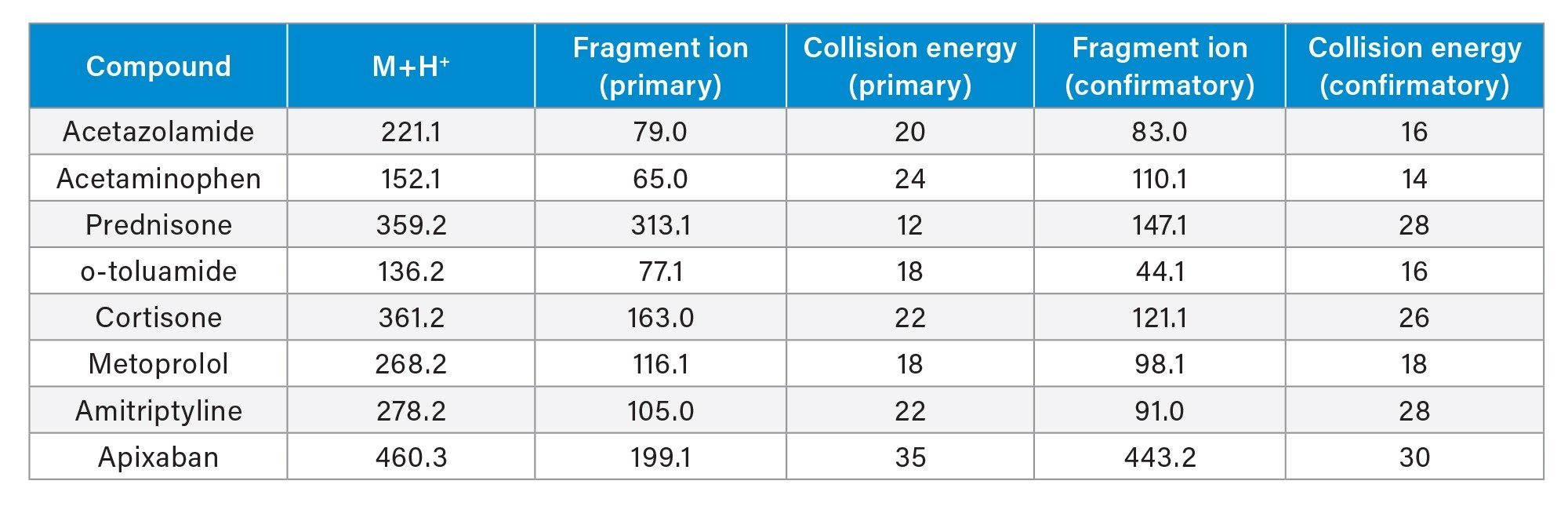
A stock solution containing a mixture of small molecule pharmaceuticals (10 µg/mL) was prepared in methanol from individual 1.0 mg/mL concentrated analyte stock solutions. This solution was then used to prepare a 100 ng/mL working analyte stock solution in water, which was then used to assess automated and manual reversed-phase (RP) SPE analytical performance. Neat recovery experiments were conducted using the 100 ng/mL analyte mix working stock solution in 1, 3, and 6 cc Oasis HLB SPE cartridges, as well as in the μElution and macro 96-well Oasis HLB SPE plates (N=4 per SPE format), using a simple 3-step SPE protocol (load, wash, elute). Information regarding the basic RP SPE procedure can be found in the Waters SPE Wall Chart (p/n 720001981). Briefly, an aqueous 4% phosphoric acid solution was directly loaded to the SPE plate or cartridge followed by the addition of the spiked 100 ng/mL analyte solution and mixed using a six-time pipette mixing step. The 4% aqueous phosphoric solution is often used to ensure sufficient disruption of the target analytes from the biological matrix proteins. This pretreated sample was loaded on the SPE cartridge or plates. Following sample loading, the SPE device was washed with a 5% methanol solution, and then eluted into appropriate collection vessels using a methanol solution. Samples were then diluted with water to ensure compatibility with the initial LC conditions. Respective volumes used for the sample pretreatment, SPE extraction, and sample dilution are provided in Table 1. Following SPE extraction, resulting samples were covered with screw caps or a 96-well cap mat and vortexed. Subsequent detection and analysis of the extracted samples was performed by LC-MS/MS using an ACQUITY UPLC I-Class System coupled to the Xevo TQ-XS Mass Spectrometer. Full LC-MS conditions can be found in the Experimental Section and using the MS MRM parameters listed in Table 2 for detection and analysis of the pharmaceuticals.
SPE Automated Extraction
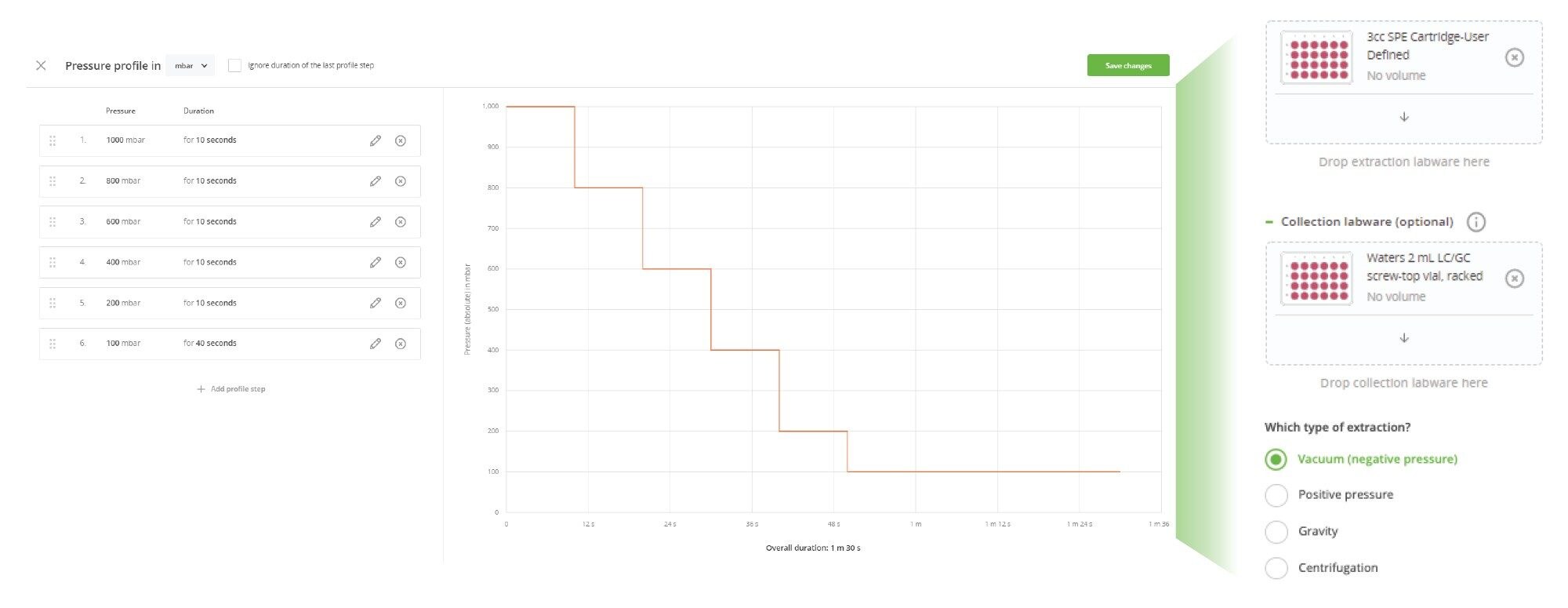
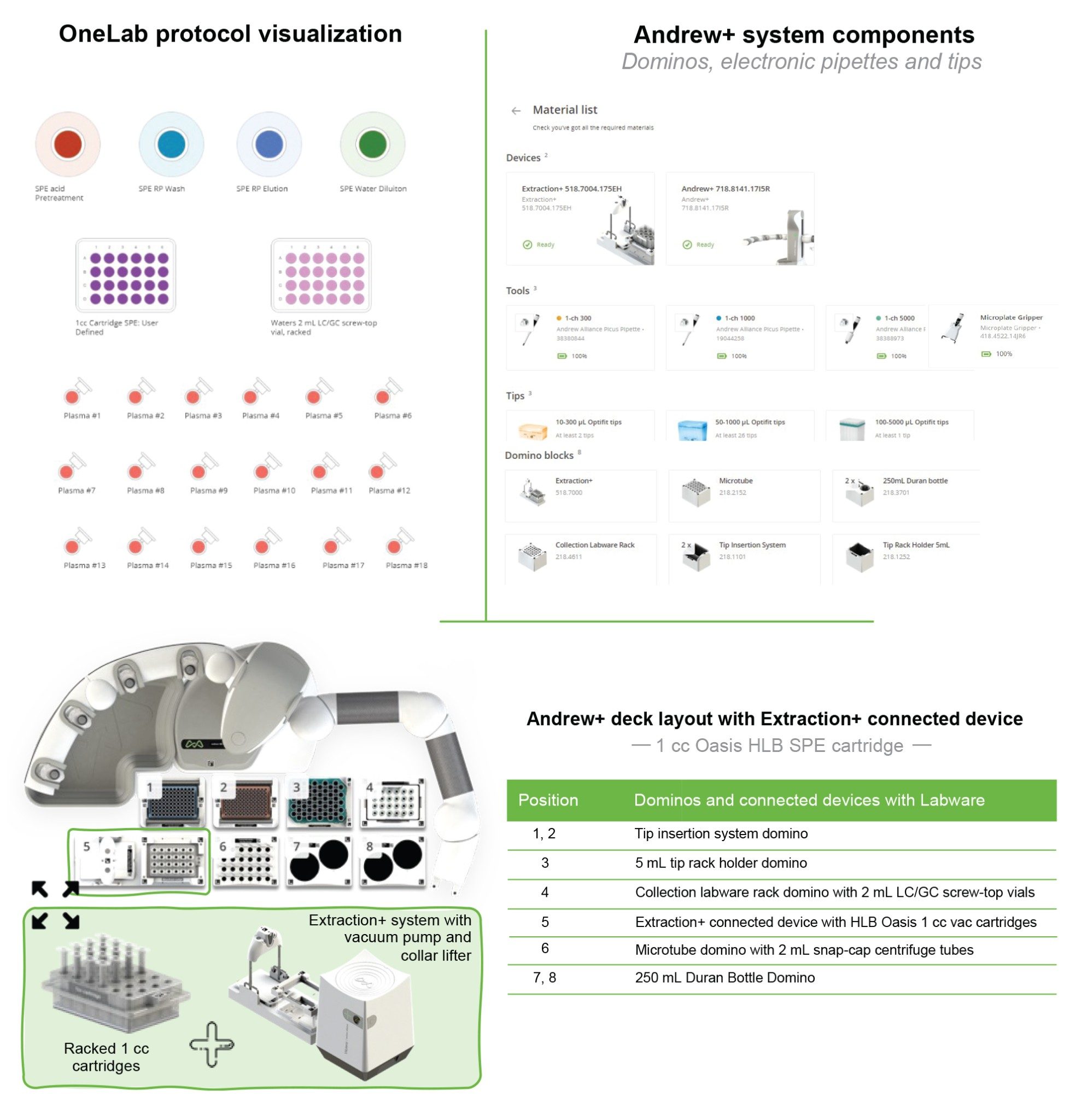
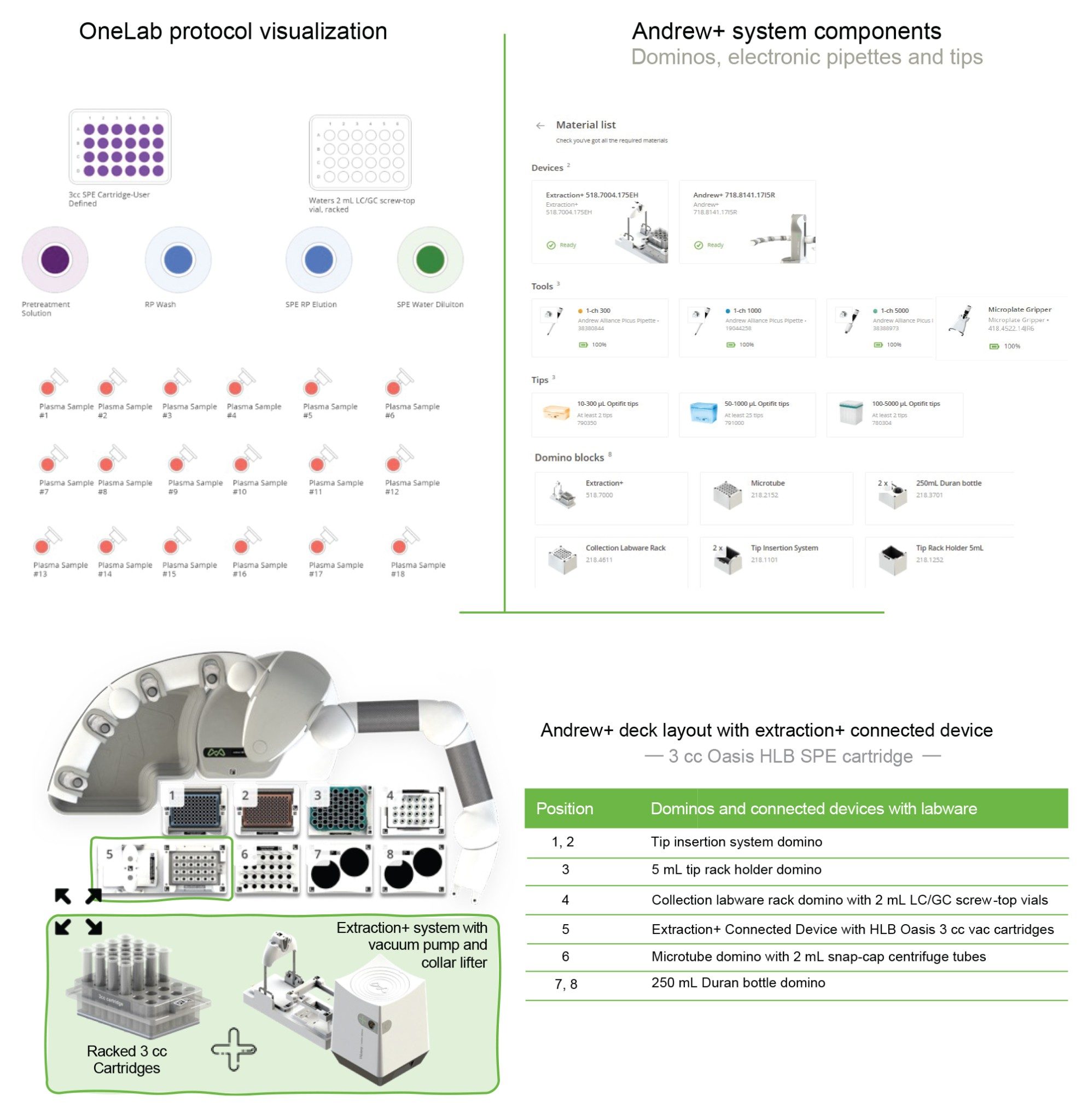
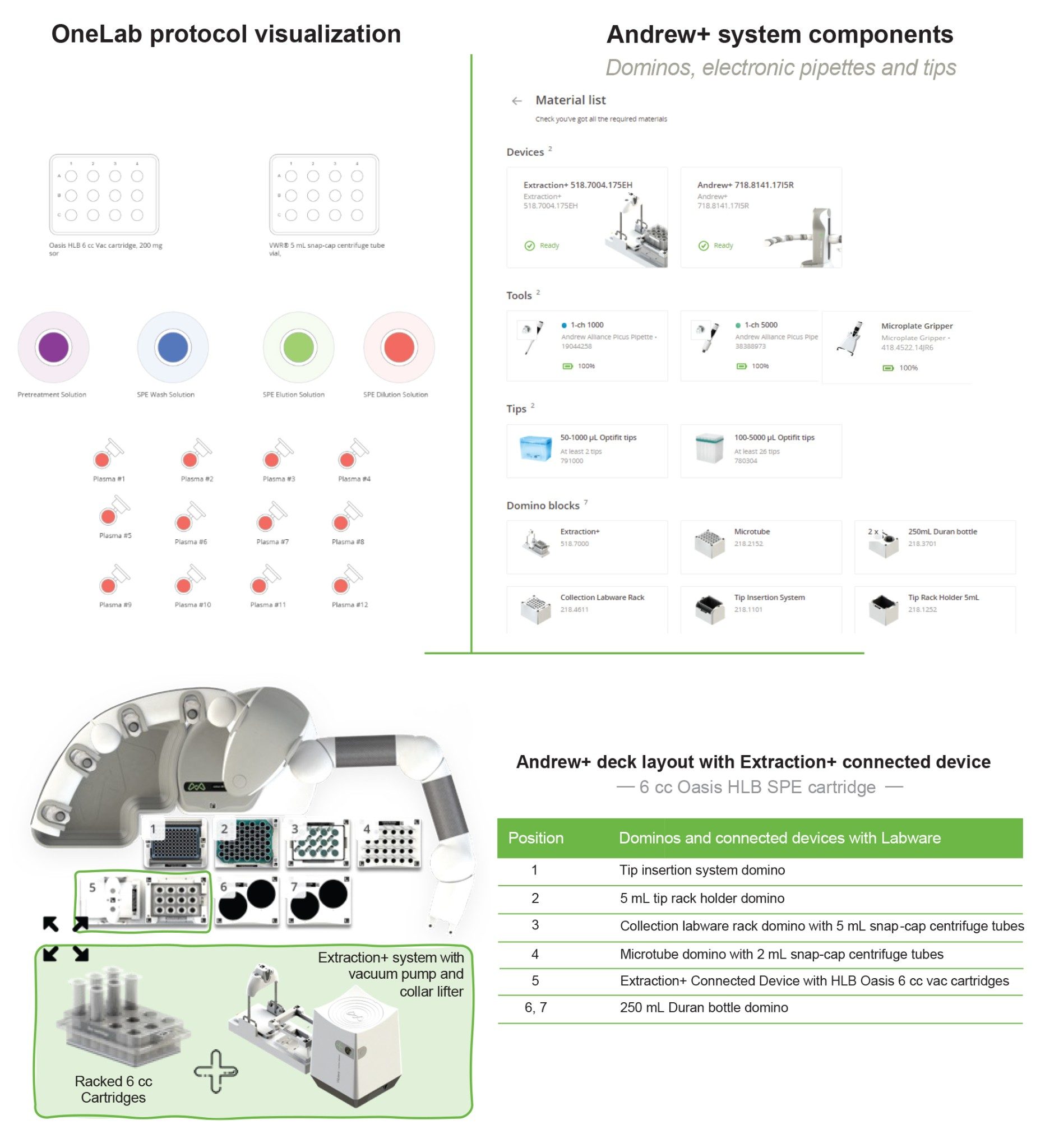
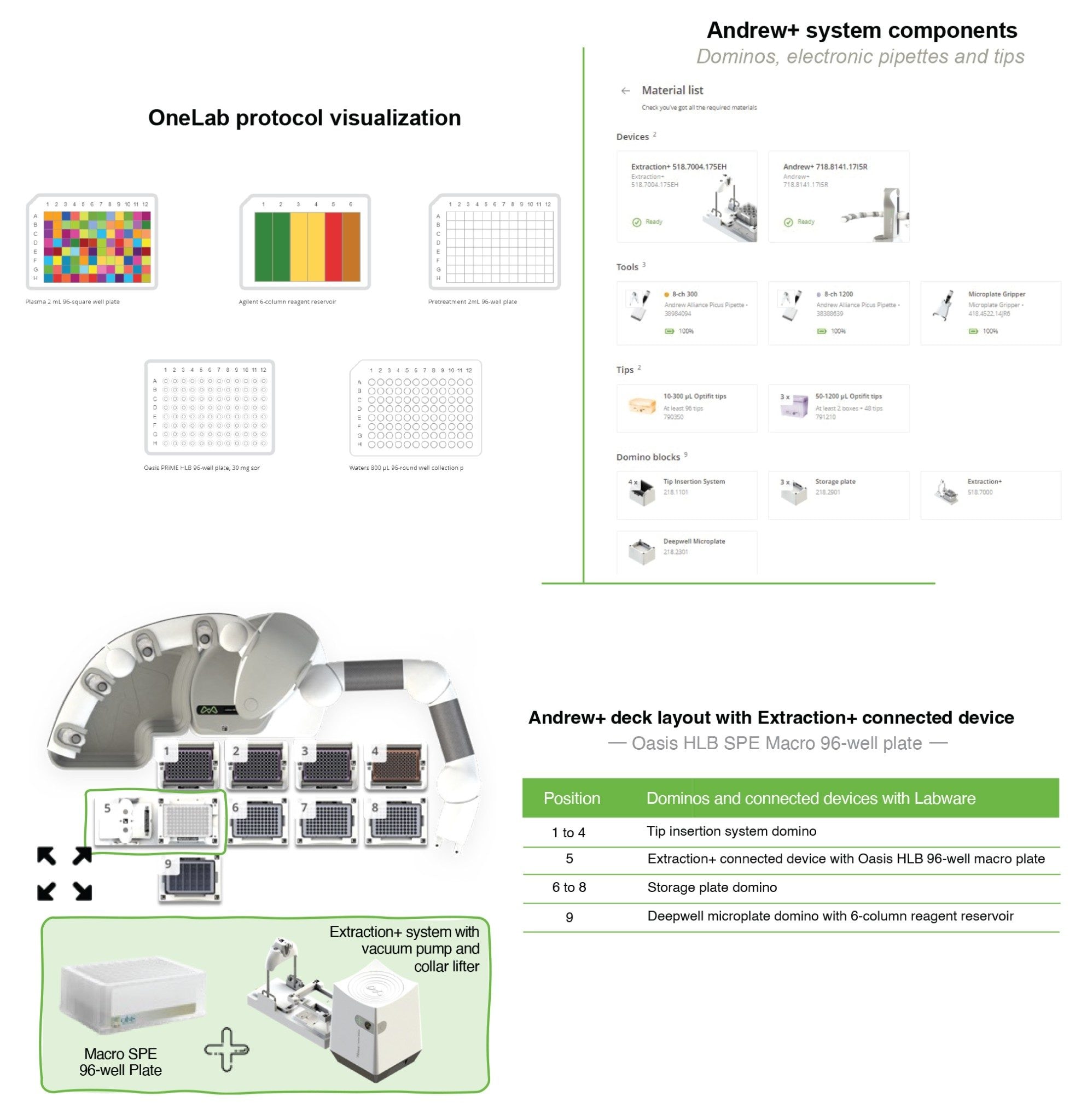
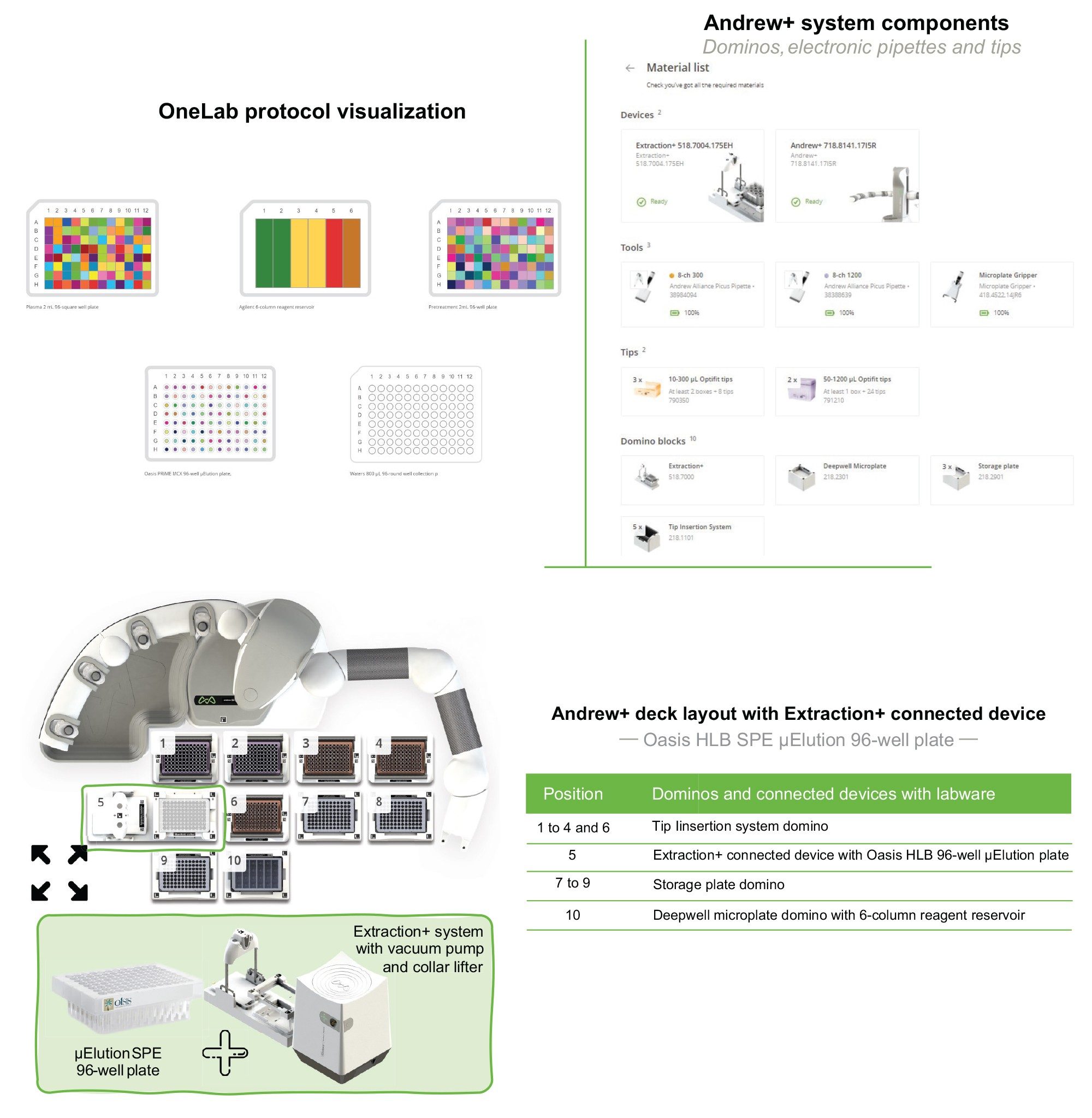
The Andrew+ configured with the Extraction+, controlled by the OneLab Software, was used to carry out all automated SPE evaluations and is shown in Figure 2. A negative vacuum using a pressure step ramp controlled via OneLab Software was applied to the SPE steps. An illustration of a typical pressure profile defined in the OneLab protocol is shown in Figure 3. Visual representation of the OneLab protocols used for the SPE cartridge and plate evaluation of Extraction+ performance, and available for download from Andrew Alliance OneLab library, are shown in Figures 4–8. These protocols were created using the SPE conditions in Table 1 for the various SPE cartridge and plate formats. Contained in these figures, are the OneLab protocols with protocol visualization and drag-and-drop method creation, key information such as system components (e.g., dominos, connected devices, pipettes, pipette tips, bottles, tubes, etc.), lab consumables, and the Andrew+ deck layout for easy automated method implementation and execution with the Extraction+.

OneLab Protocol 1 | Oasis RP-HLB 1 cc Cartridge SPE
OneLab Protocol 2 | Oasis RP-HLB 3 cc Cartridge SPE
OneLab Protocol 3 | Oasis RP-HLB 6 cc Cartridge SPE
OneLab Protocol 4 | Oasis RP-HLB 96-well Macro Plate SPE
OneLab Protocol 5 | Oasis RP-HLB 96-well µElution Plate SPE
Results and Discussion
Extraction+ is the new, fully automatable SPE system solution used in combination with Andrew+, which eliminates the need for user intervention during SPE sample preparation and extraction. The Extraction+ device is comprised of two modules, an SPE manifold and a vacuum pump, both controlled via the OneLab Software. An image of the Extraction+ configured with the Andrew+ is shown in Figure 2. Key features of the Extraction+ include:
- Compatibility to perform SPE in both cartridge and plate formats,
- Liquid dispensing, collection, and evacuation into plates, tubes, or bottles,
- Flow-through waste collection,
- Full vacuum pressure control, defined within the OneLab protocol, for evacuation of the liquid during the SPE method execution (Figure 3).
The labware gripper installed on Andrew+ facilitates the movement of waste and sample collection vessels to and from the Extraction+ vacuum manifold, while the collar lifter on the Extraction+ manifold, moves the extraction manifold collar with the SPE device into a ‘parking spot’ (idle position) during the insertion of the collection vessels onto the vacuum manifold with the labware gripper.
Both Andrew+ and Extraction+ are controlled through the user-friendly, cloud-based OneLab Software. The Extraction+ when used with Andrew+, can carry out the liquid handling and the sample extraction fully automated (no manual intervention), providing complete walk-away sample preparation, whilst ensuring robust and reliable sample preparation from lab-to-lab, day-to-day, and user-to-user.
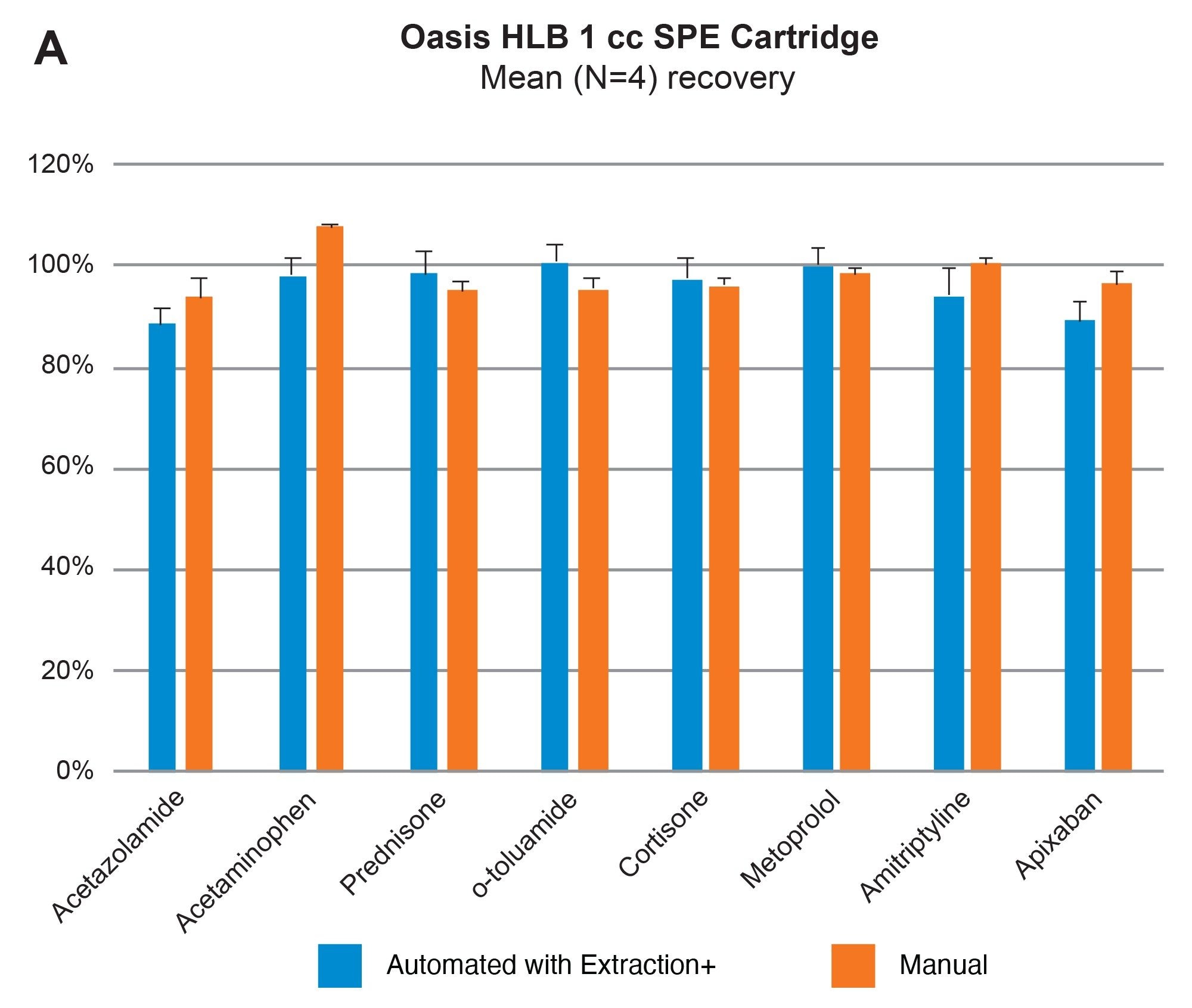
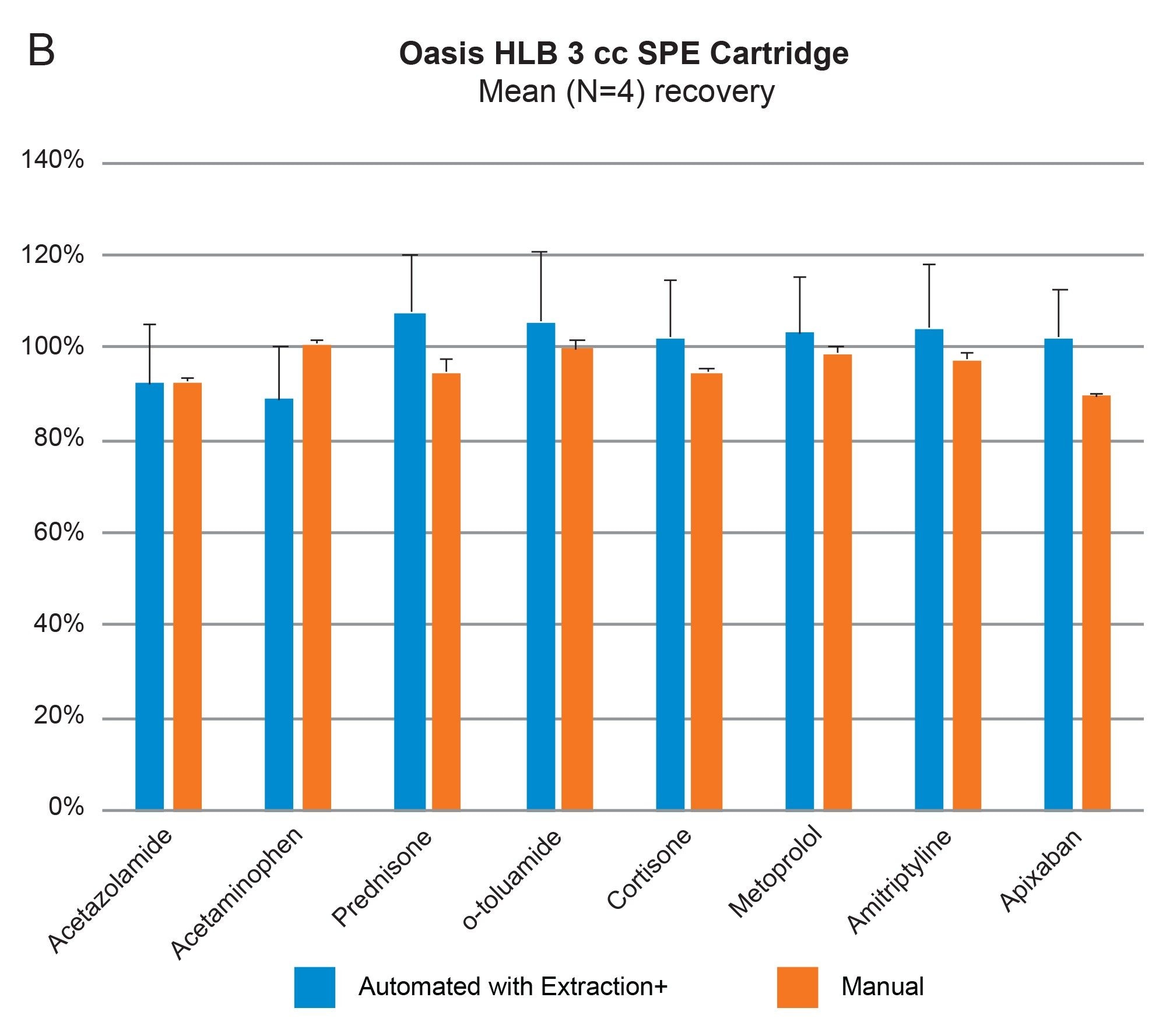
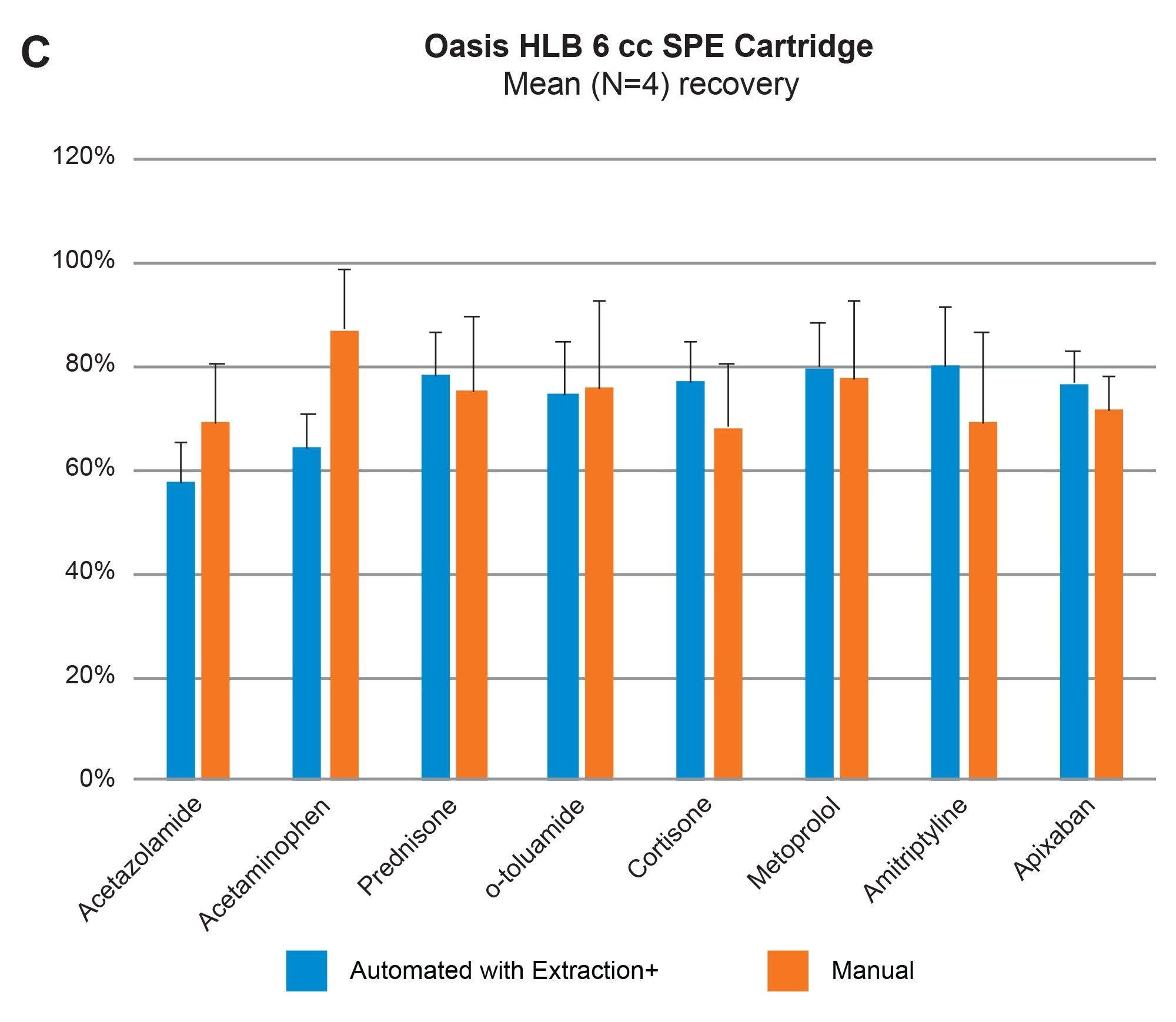
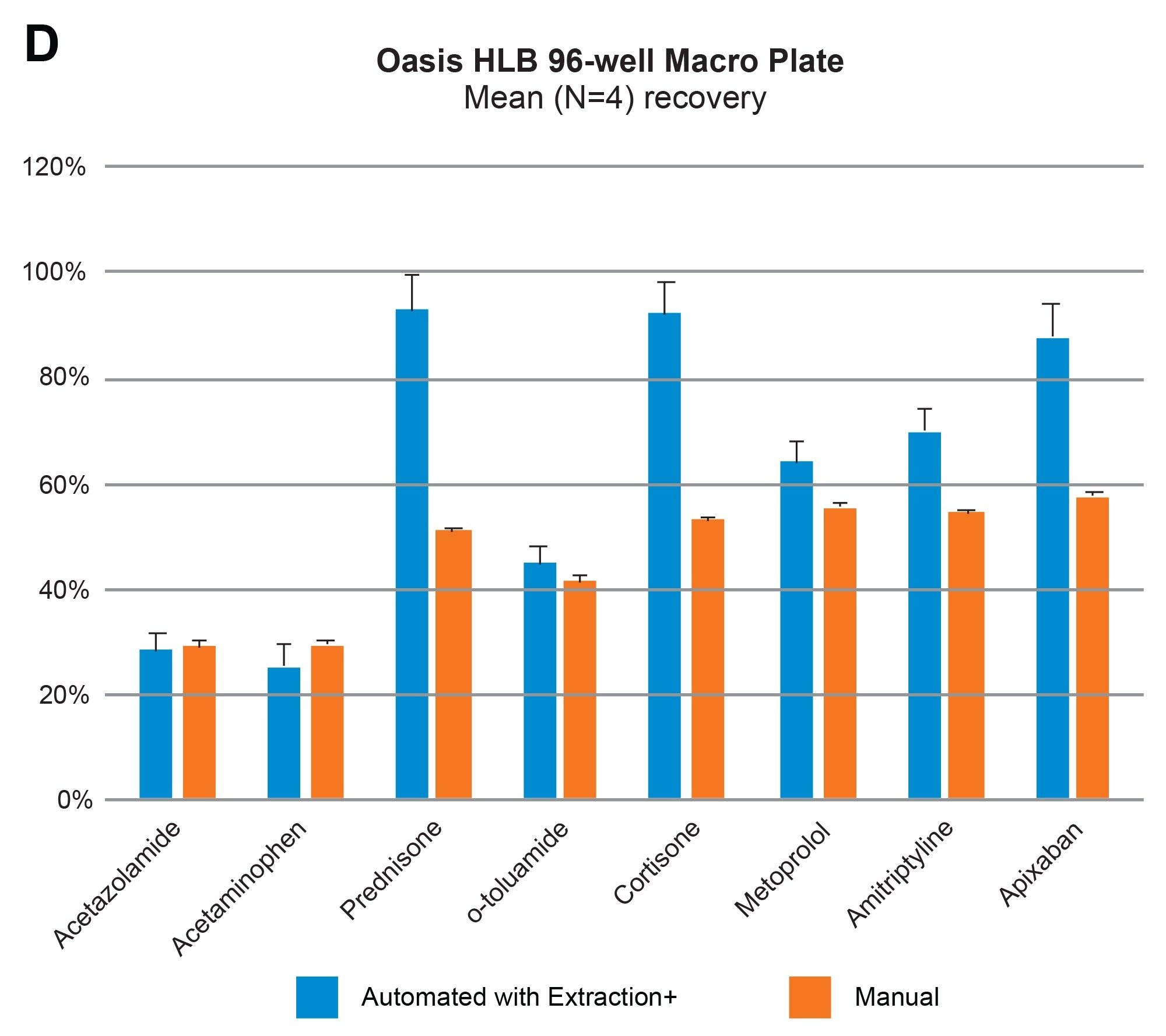
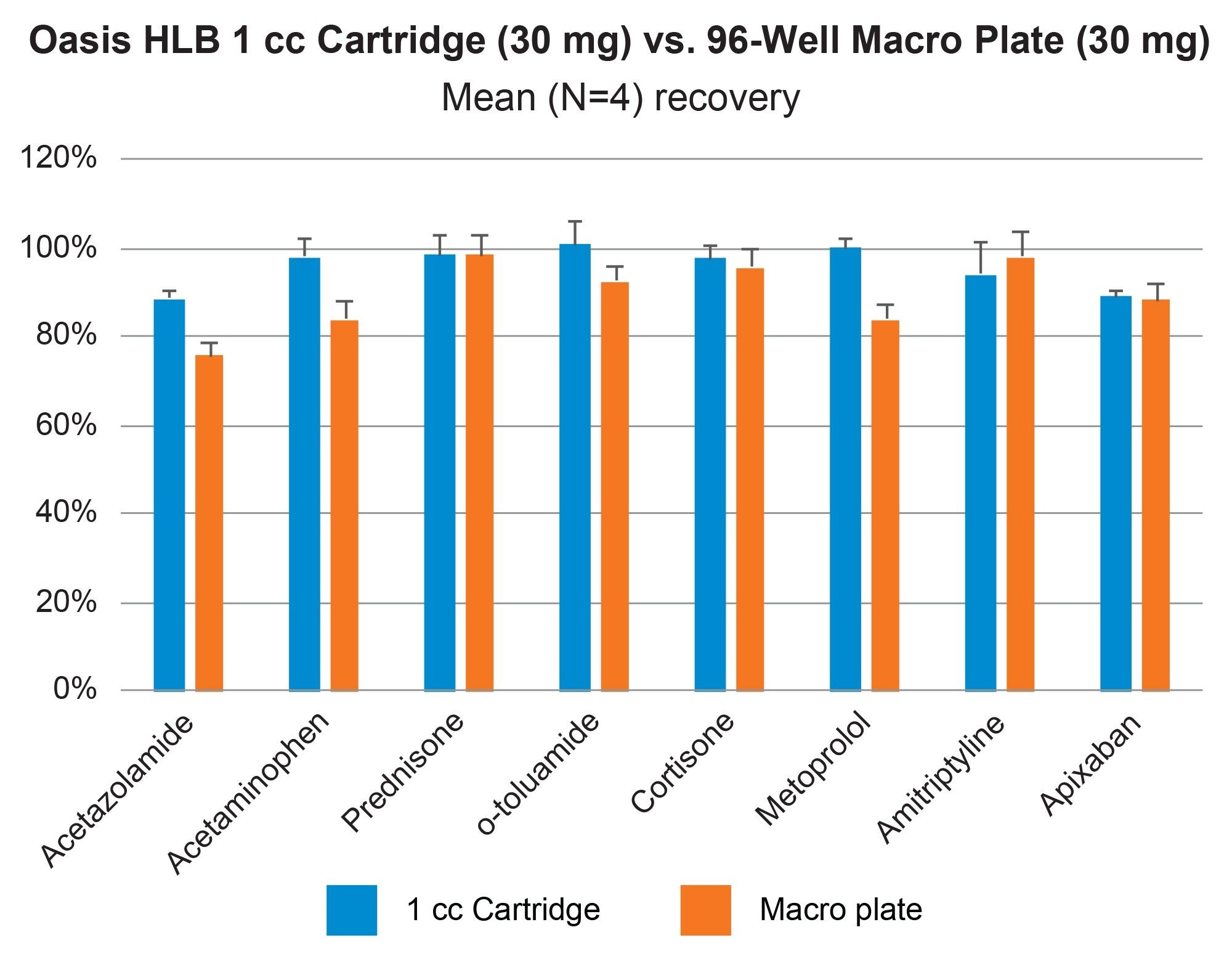
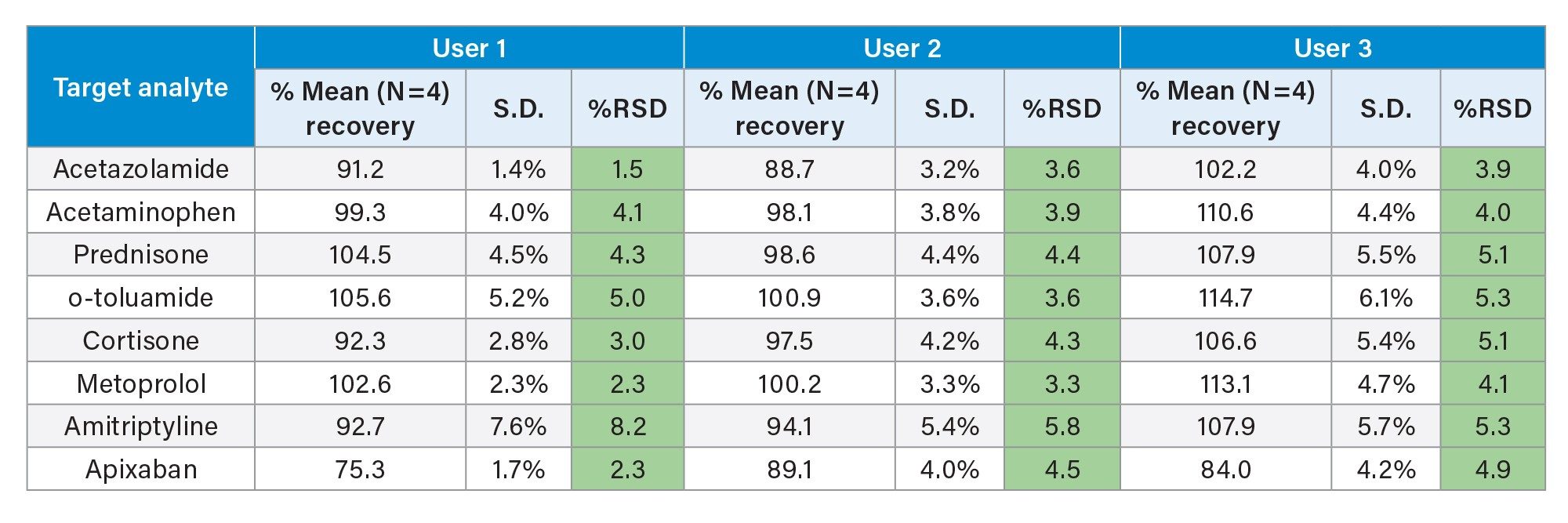
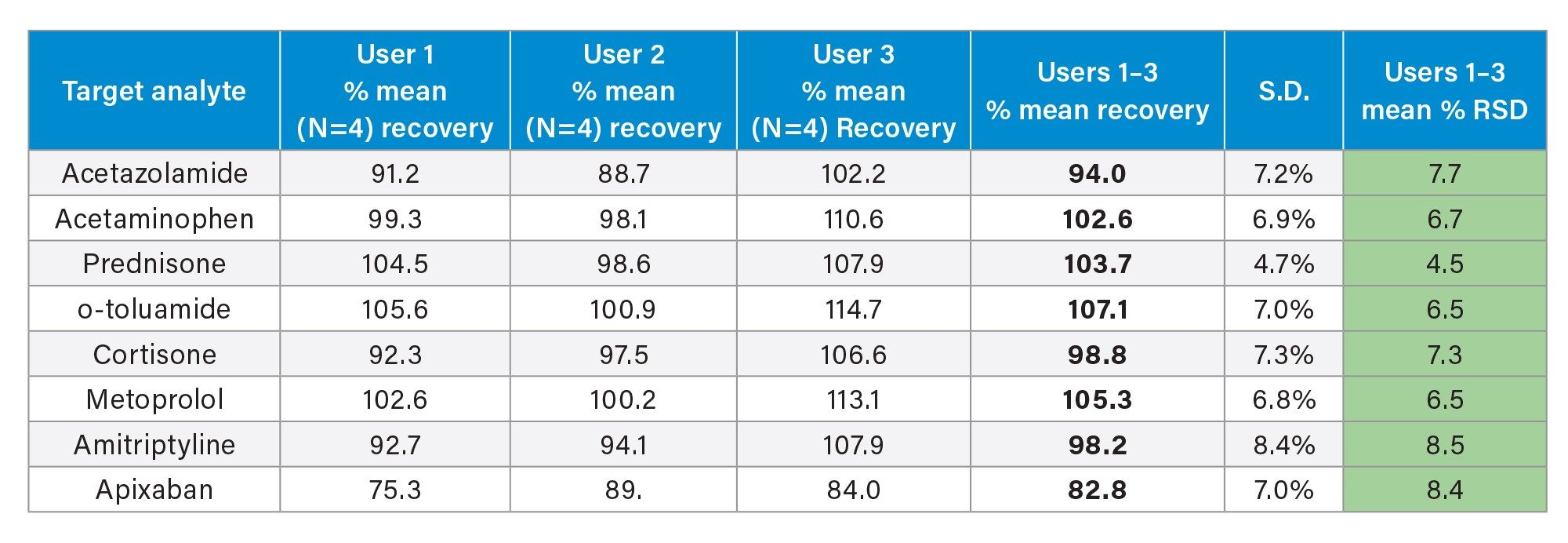
Comparable SPE extraction performance using the Andrew+ configured with the Extraction+ using 1, 3, 6 cc RP Oasis HLB cartridges and 96-well Macro plate as compared to manual SPE is shown in Figure 9, panels A–D. Furthermore, robust and repeatable, automated SPE performance in 1 cc Oasis HLB cartridges is illustrated in Tables 3 and 4, highlighting single digit intra/inter-assay performance (mean recovery and RSD). Figure 10 highlights comparable Oasis HLB automated SPE extraction performance for both cartridge and 96-well plate formats, using the same sorbent bed size (30 mg). Collectively these results demonstrate highly flexible and reproducible Oasis HLB SPE sample preparation in both cartridges and plates, with the developed SPE protocols created in the OneLab Software and executed with the Andrew+ Pipetting Robot configured with the Extraction+ connected device.
Conclusion
This application highlights fully automated SPE sample preparation in cartridge and plate formats, leveraging the capabilities of the newly introduced connected device, Extraction+ configured on Andrew+. In combination with its fully software-controlled pipetting, vacuum pump control, and liquid transfer in collection plates, tubes, and bottle, the use of the static and core grippers with Extraction+ facilitates movement to and from the extraction manifold, eliminating the need for user intervention during the SPE sample preparation.
The OneLab protocols for the described SPE cartridges and plate formats are downloadable from the OneLab Library providing ease of use and transferability across Andrew+ systems, which allows for easy implementation of the same preparation procedure across users in the same lab or between connected labs. Andrew+ with Extraction+ simplified and streamlined sample preparation and extraction procedures, maximizing productivity, reducing errors, and ensuring the overall analytical performance of a typical bioanalytical LC-MS workflow.
720007711, September 2022