
This is an Application Brief and does not contain a detailed Experimental section.
Using UPC2 with smaller particle columns, a rapid method for chiral analysis of clenbuterol was developed. The analysis was completed in less than three minutes, allowing for high throughput analysis. The method was also reproducible over several injections and utilized mobile phases compatible with mass spectrometry detection for possible analysis in bioanalytical studies.
Fast analysis in less than 3 minutes
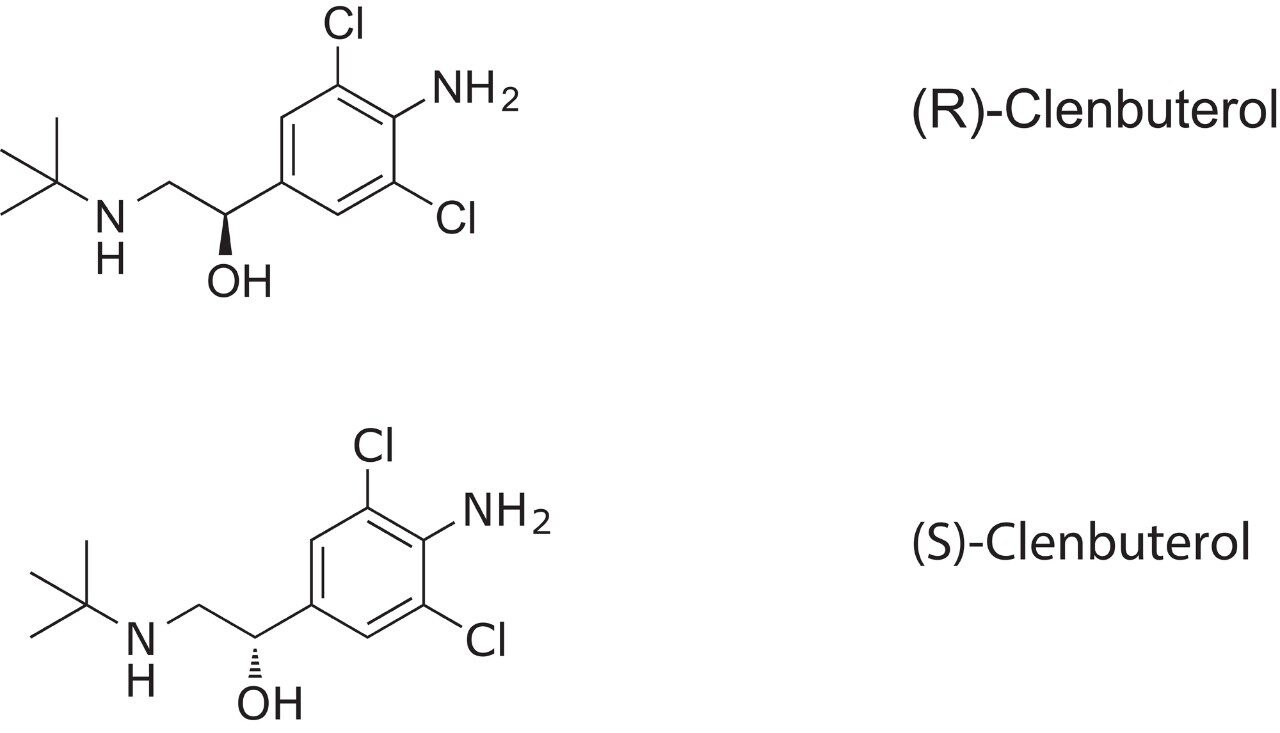
Clenbuterol is a drug used by people with chronic breathing disorders, such as asthma, as a bronchodilator to make breathing easier. Clenbuterol is more potent and longer-lasting as a stimulant than other compounds. Dosage is typically in the range of 2 to 40 mg per day, thus sensitive methods of analysis are needed to separate the enantiomers of clenbuterol at a low level.
|
System: |
ACQUITY UPC2 with photodiode array (PDA) detection |
|
Column: |
CHIRALPAK IA, 4.6 x 100 mm, 3 μm |
|
Column temp.: |
40 °C |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
MeOH with 0.5% CH3COONH4 |
|
Isocratic conditions: |
85% A, 15% B |
|
Flow rate: |
2 mL/min |
|
Back pressure: |
1500 psi |
|
Detection: |
UV 297 nm, compensated 350 to 450 nm |
|
Injection volume: |
2 μL for 0.2 mg/mL, 6 μL for 0.002 mg/mL |
|
Sample prep: |
0.1 mg/mL and 0.001 mg/mL each enantiomer in 1:1 EtOH/heptane |
|
Vials: |
Waters Maximum Recovery Vials |
|
Data management: |
Empower 3 Software |
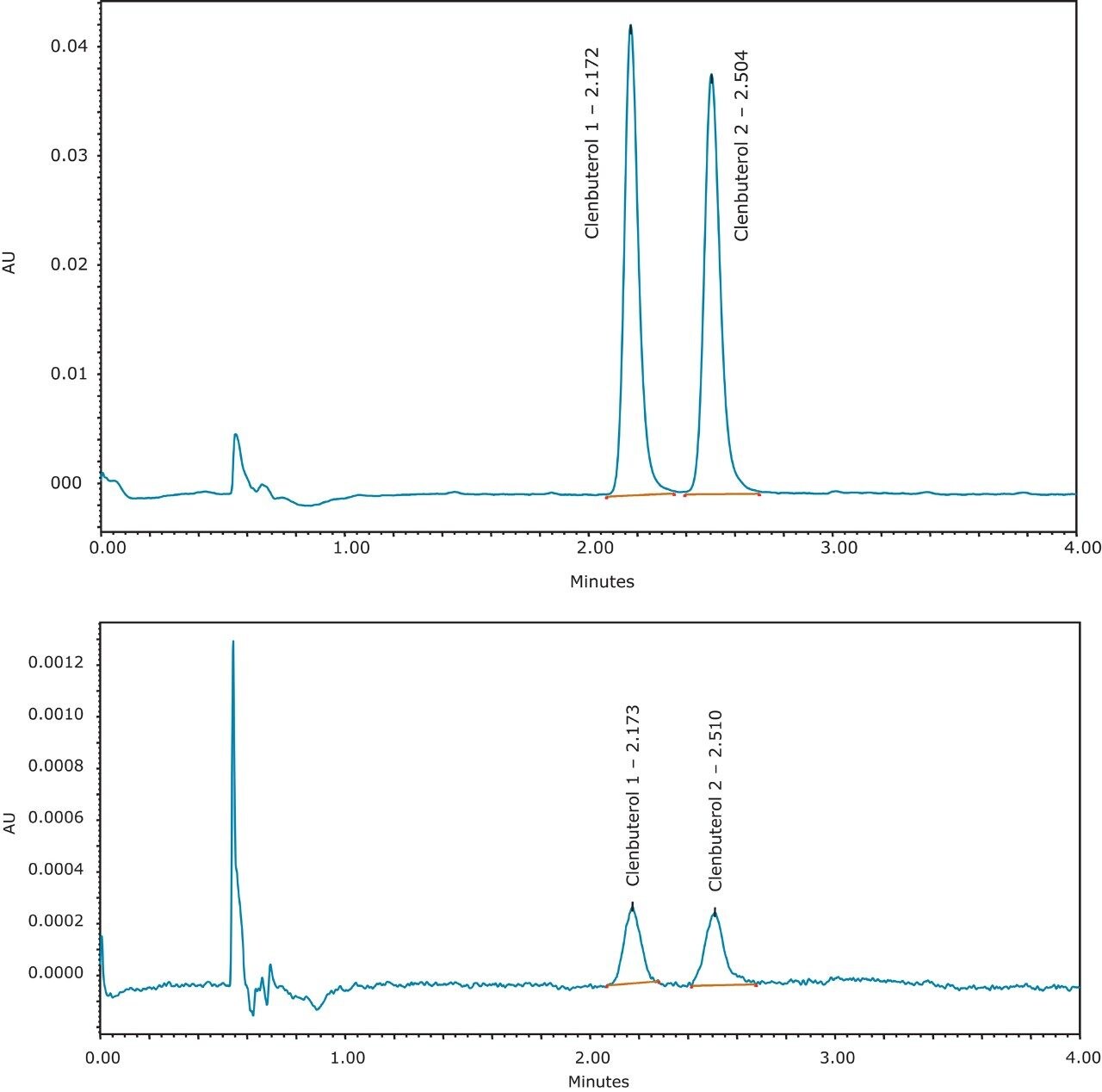
Using UPC2 with smaller particle columns, a rapid method for chiral analysis of clenbuterol was developed. The analysis was completed in less than 3 min, allowing for high throughput analysis. The limit of quantitation (LOQ) was less than 1 µg/mL using UV detection for chiral analysis at low concentrations. The method was also reproducible over several injections and utilized mobile phases compatible with mass spectrometry detection for possible analysis in bioanalytical studies.
720004537, December 2012