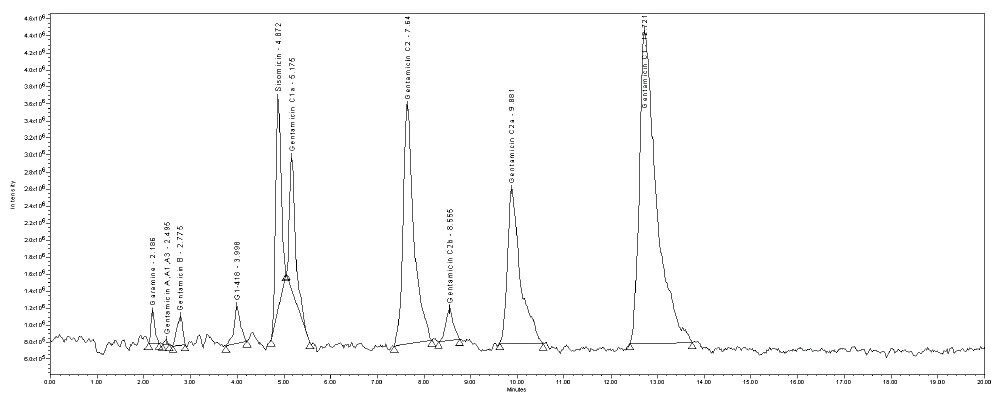
In this method, five main analytes of Gentamicin (C1, C1a, C2, C2a, and C2b) and related impurities of sisomicin, G1-418, garamine, gentamicin B, and gentamicin A, A1, A3 are separated within 35 minutes using an ACQUITY UPLC H-Class System with an ACQUITY QDa Mass Detector. The ACQUITY QDa Mass Detector is robust, reliable, and requires minimal user setup optimization, calibration, or adjustment. It integrates with current LC, ACQUITY UPLC, ACQUITY UPC2, and purification systems.
Separation of five active components of gentamicin and its related impurities within a 35-minute run time on ACQUITY UPLC H-Class System with ACQUITY QDa Detector.
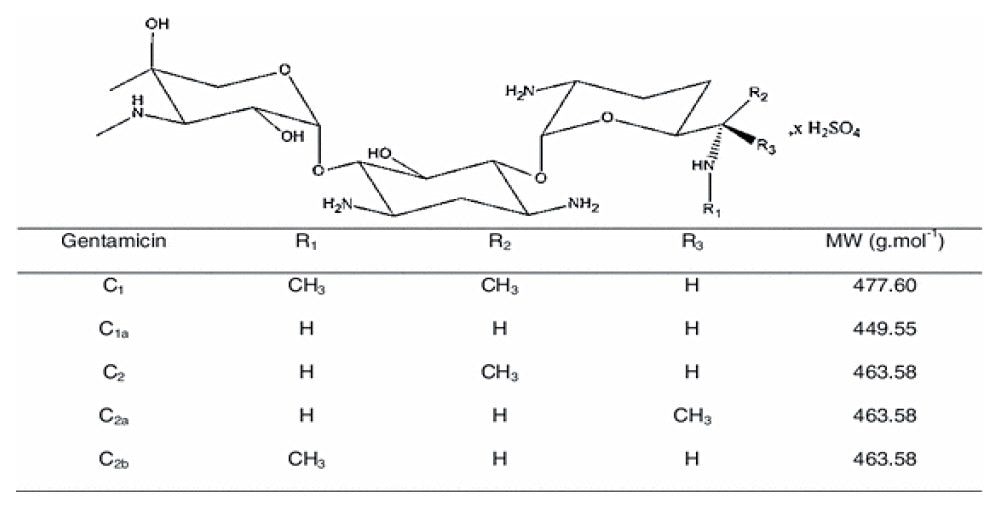
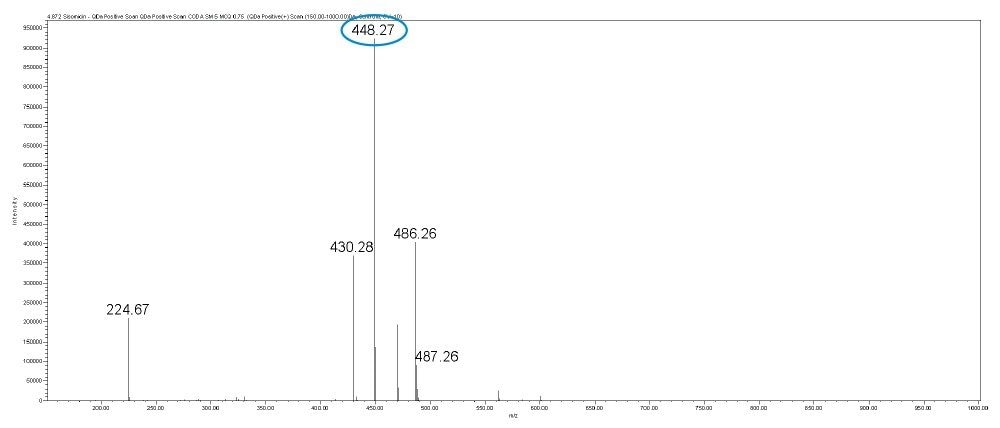
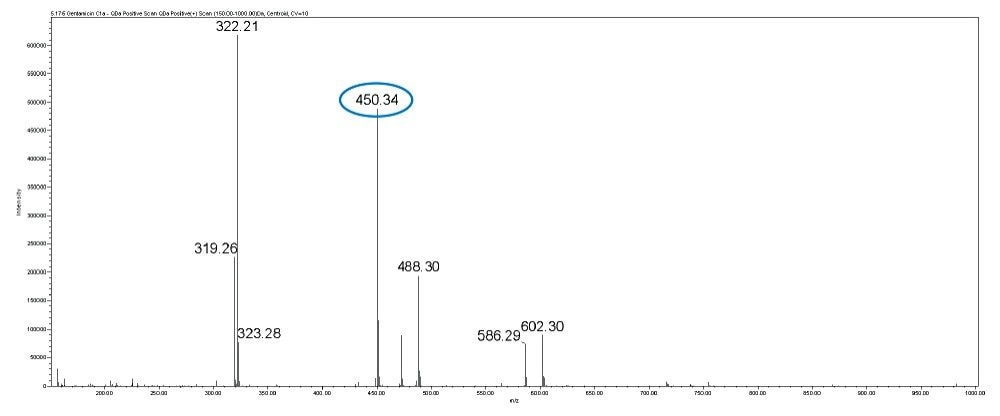
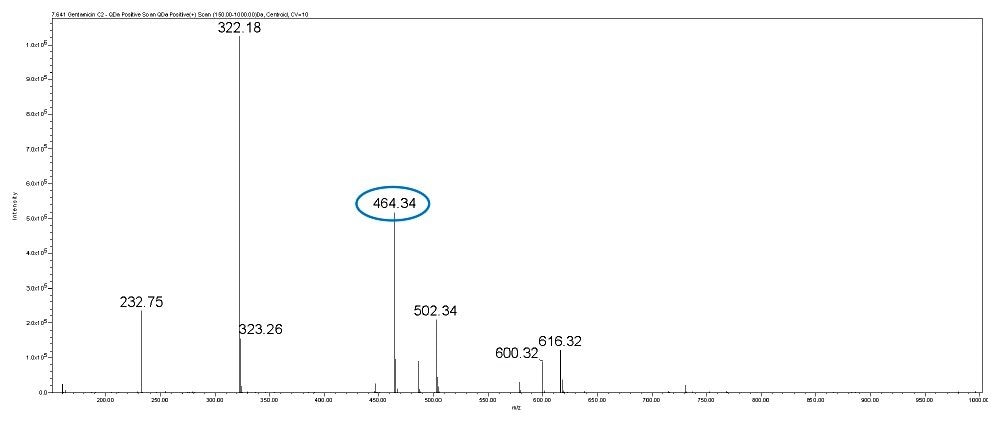
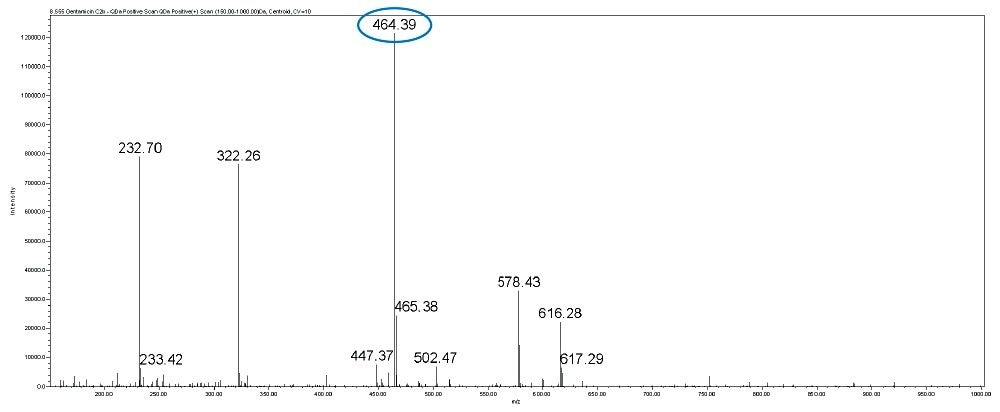
Gentamicin is an aminoglycoside antibiotic (AG) produced by Micromonospora purpurea and is widely used for the treatment of serious infections caused by both gram-negative and gram-positive bacteria. It is a mixture consisting of five major components, designated C1, C1a, C2, C2a, and C2b together with numerous minor components including A, B, B1, and X2. Gentamicins are basic, water-soluble, relatively stable, structurally and closely related compounds without UV absorbing chromophores. This makes the HPLC analysis more difficult and challenging. Detection techniques like UV are not sensitive enough to detect low levels of related compounds of gentamicin.
Mass spectrometry is the technique of choice for the detection of aminoglycosides including gentamicin because of its high sensitivity and identification of compounds. There are no derivatization steps involved for this analysis technique.
In this experiment we have developed a 35-minute method for the qualitative and quantitative analysis of gentamicin sulfate and its related impurities on an ACQUITY UPLC System with an ACQUITY QDa Mass Detector.
The ACQUITY QDa Mass Detector is robust, reliable, and requires minimal user setup optimization, calibration, or adjustment. It integrates with current LC, ACQUITY UPLC, ACQUITY UPC2, and purification systems. This mass spectral information integrates seamlessly into the same workflow. The ACQUITY QDa Detector offers extended sample detection to quantify compounds with no UV response and is compatible with Empower Chromatography Data Software.
|
System: |
ACQUITY UPLC H-Class with ACQUITY QDa Detector |
|
Column: |
Atlantis T3, 3 μm, 4.6 mm × 150 mm (p/n: 186003729) |
|
Flow rate: |
0.6 mL/min |
|
Buffer preparation: |
0.2% TFA in water adjusted pH 2.3 with ammonia solution |
|
Mobile phase A: |
99.5% (buffer):0.5% (ACN+IPA:[1:1]) |
|
Mobile phase B: |
100% methanol |
|
Column temp.: |
25 °C |
|
Sample temp.: |
5 °C |
|
Injection volume: |
1 μL |
|
Sample concentration: |
100 μg/mL |
|
Wash and purge solvent: |
1:1 water:acetonitrile |
|
Seal wash: |
9:1 water:methanol |
|
Diluent: |
Water |
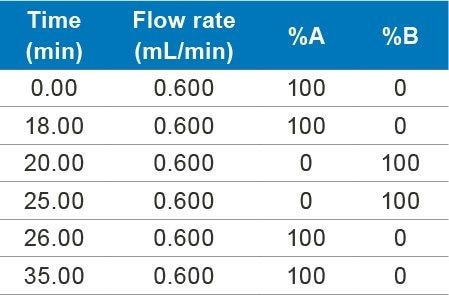
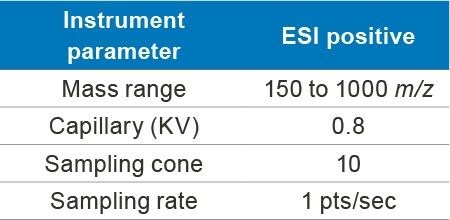
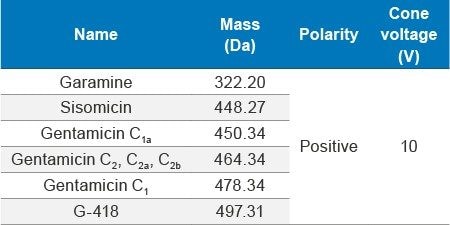
Accurately weighed 10 mg of gentamicin sulfate (API) and dissolved in 2 mL of water as a standard stock solution of 5000 µg/mL concentration. Working solutions were prepared by appropriate dilutions from stock.
Accurately weighed 6 mg of sisomicin and dissolved in 10 mL of water as a stock solution of 600 µg/mL concentration. Working solutions were prepared by appropriate dilutions from stock.
Sample solutions of injections containing 20 mg/2 mL of gentamicin sulfate, were prepared by diluting the sample with water to a working concentration of 100 µg/mL of total gentamicin.
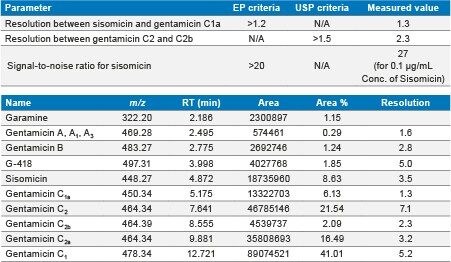
Injected API spiked with sisomicin standard and observed all five analytes and impurities.
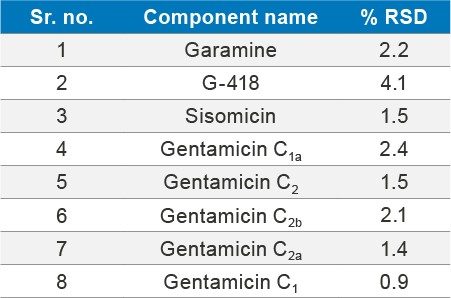
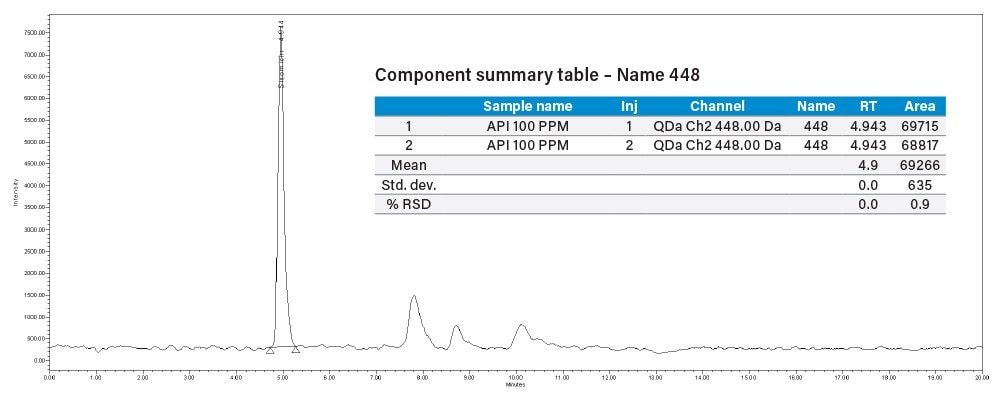
The reproducibility test was performed with API of 100 µg/mL concentration spiked with 50 µg/mL of sisomicin impurity, the %RSD observed for the eight components in SIR method were within 5%.
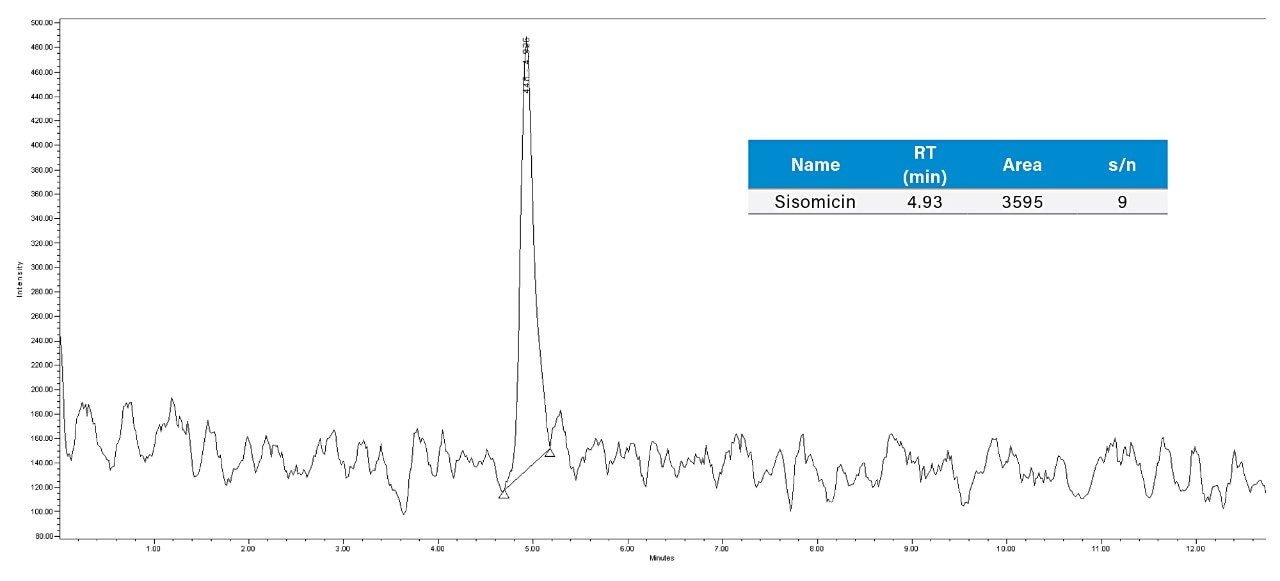
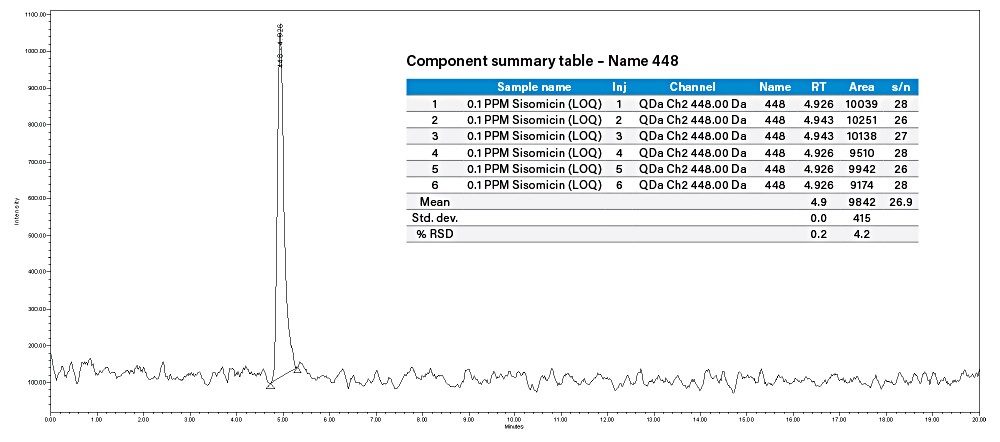
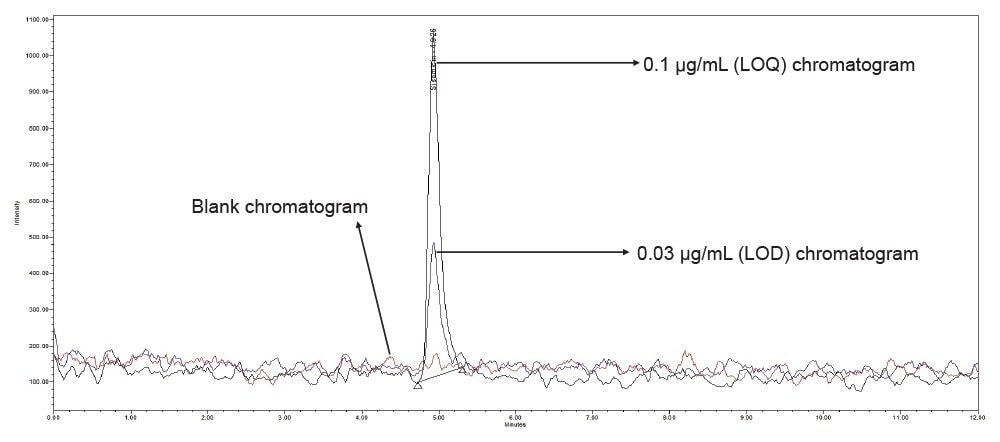
Injected 0.03 µg/mL (LOD) and 0.1 µg/mL (LOQ) concentration of sisomicin standard solution and observed that the S/N is 9 for sisomicin peak in 0.03 µg/mL and 27 is for 0.1 µg/mL.
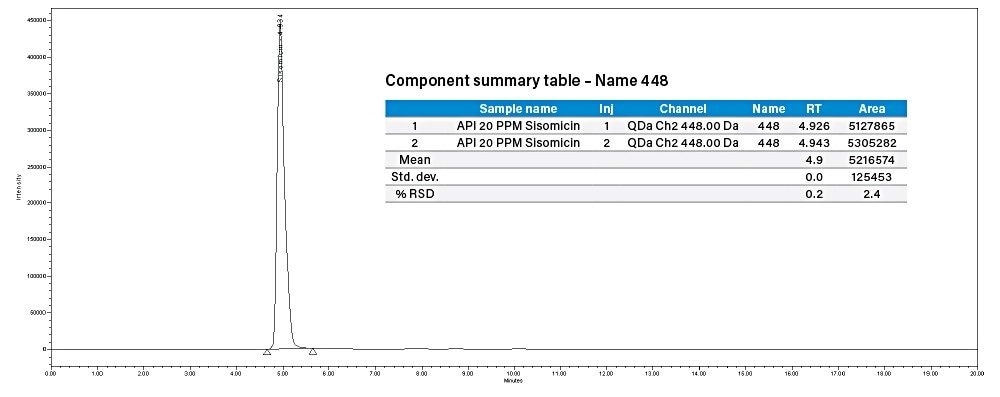
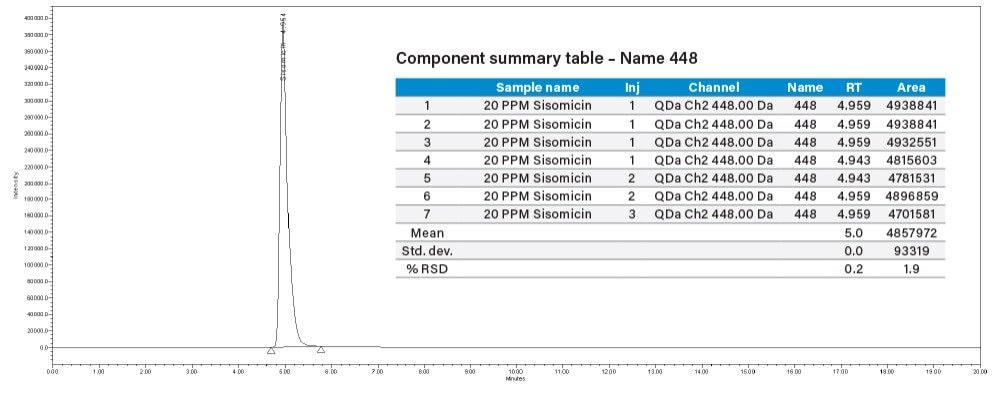
Injected 20 µg/mL concentration of sisomicin individual standard and 20 µg/mL sisomicin solid powder spiked with 100 µg/mL concentration of API gentamicin. 105.96% recovery was observed.
% Recovery = (Sisomicin peak area in spiked solution – API solution)/[sisomicin std area (20 μg/mL)]
= (5216574-69266)/4857972
= 105.96%
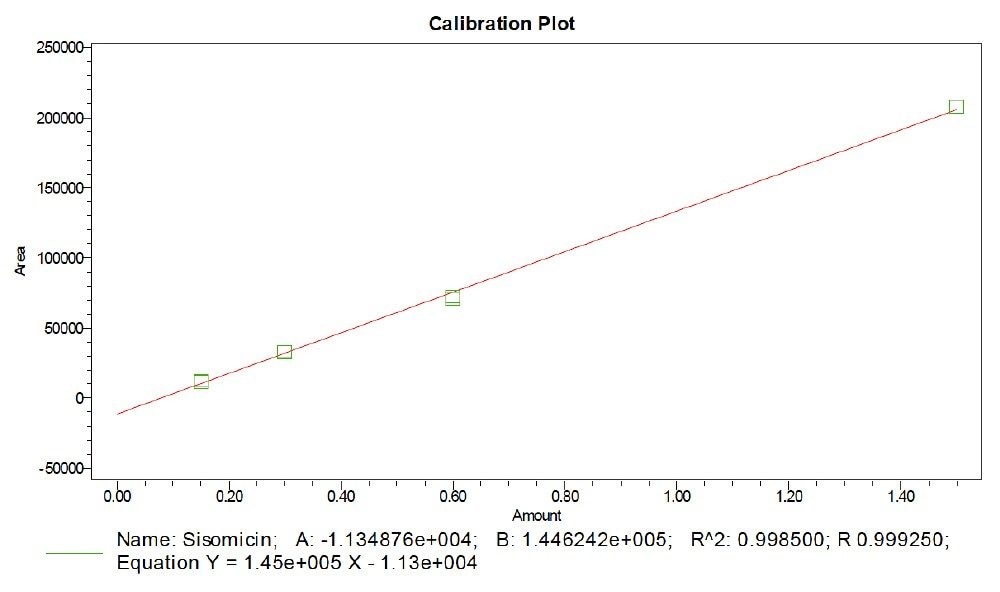
Prepared linearity solutions of sisomicin with different concentrations of 0.15 µg/mL, 0.3 µg/mL, 0.6 µg/mL, and 1.5 µg/mL solutions and plotted calibration curve, observed R value is 0.999 and R2 value is 0.998.
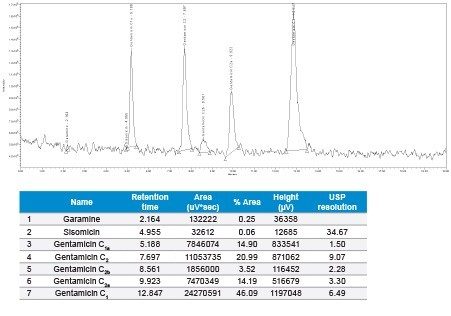
Diluted gentamicin sulfate USP injectable sample (Lot No: 6110501 – 20 mg/2 mL) to 100 µg/mL concentration and injected, observed the related impurities in SIR method.
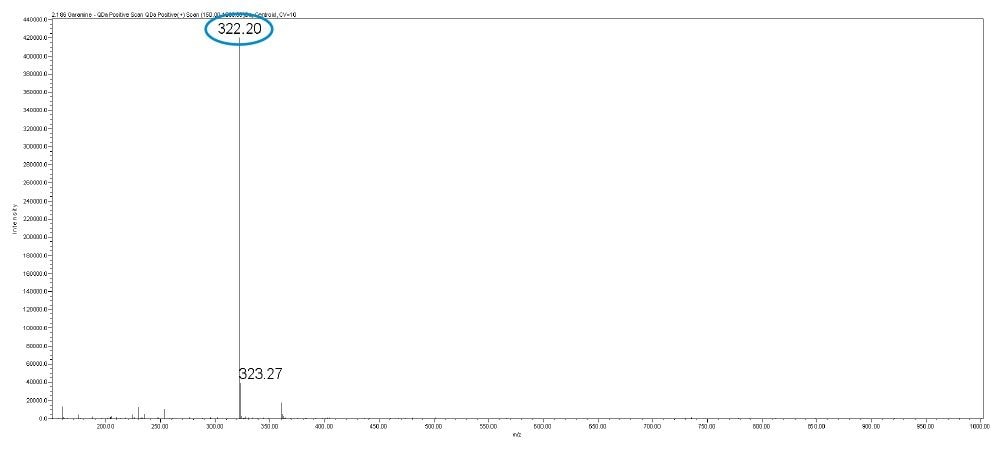
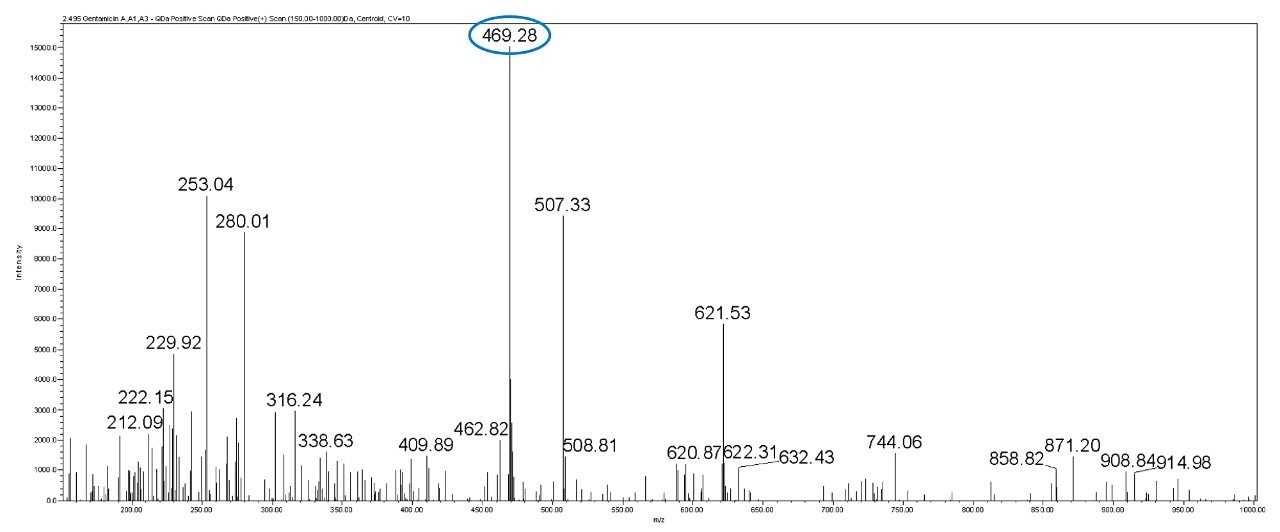
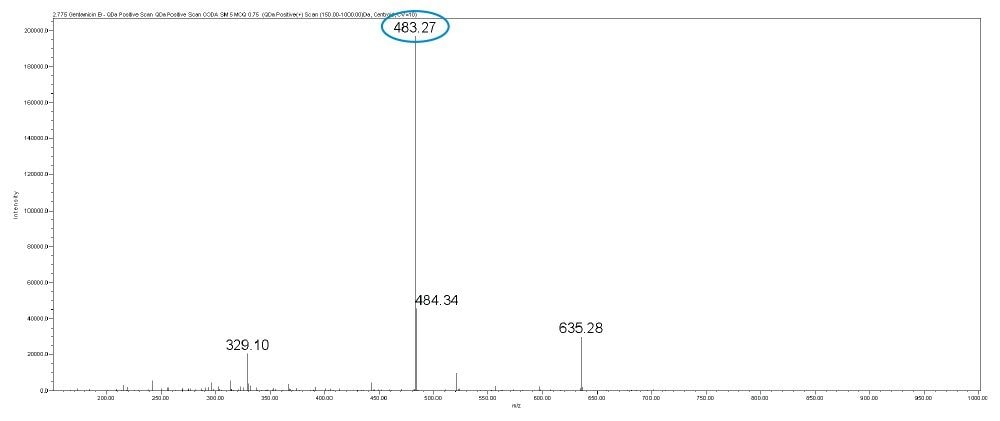
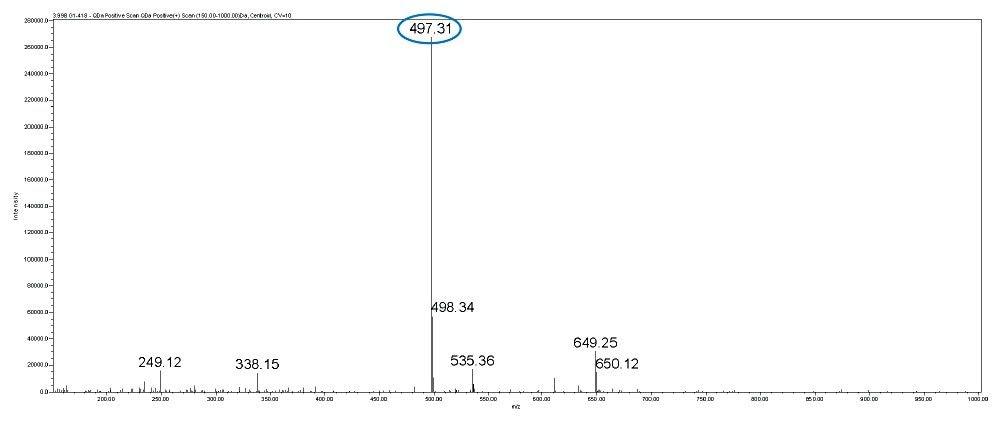
In this method, five main analytes of Gentamicin (C1, C1a, C2, C2a, and C2b) and related impurities of sisomicin, G1-418, garamine, gentamicin B, and gentamicin A, A1, A3 are separated within 35 minutes using an ACQUITY UPLC H-Class System with an ACQUITY QDa Mass Detector.
The ACQUITY QDa Mass Detector successfully achieved high sensitivity detection for sisomicin impurity at 0.03 µg/mL as LOD and 0.1 µg/mL as LOQ. It achieved reliable results. The %RSD obtained for all gentamicin analytes and impurities sisomicin and G-418 is less than 5%.

720006597, June 2019