
Global regulatory bodies require pharmaceutical manufacturers, and contract organizations or service providers to control the presence of known and unknown impurities in drug substance and final pharmaceutical drug products. Laboratories must have the capability to identify and quantify impurities and adopt onward control strategies for them with suitable analytical techniques. This facilitates meeting regulatory requirements that protect the quality, safety and onwards supply chain of released products.
Characterizing, quantifying and monitoring pharmaceutical impurities can be challenging due to the diverse chemical nature of the analytes, complexity of sample matrices, and often extremely low or trace level detection levels necessary. Waters diverse impurity analysis solutions, including chemistry and consumables, LC and MS technologies, and enabling compliant-ready software, ensure the accurate detection and identification of unknown impurities and their routine, robust, and sensitive quantification to beyond the regulatory required levels and within the expectations of a compliant laboratory environment.
Explore N-Nitrosamines Analysis





Blog: Mutagenic Impurity Risk Assessment Throughout the Development and Manufacturing Process

Streamline workflows, improve usability and compliance, and accelerate scientific and business outcomes with waters_connect lab informatics software, a single platform for LC and LC-MS applications for quantitation, identification, and other accurate mass-based analyses.
Equip your lab with Empower Chromatography Data System (CDS) and gain advanced laboratory data management for your impurity analyses, including acquisition, processing, and reporting for liquid and gas chromatography instruments.
Promote polar compound retention and aqueous mobile-phase compatibility with ACQUITY HSS T3 Columns that are more effective than traditional trimethyl silane (TMS) end-capping to enhance column performance, method development, selectivity, and stability.
Achieve reproducible and highly selective chromatographic separations with the XSelect Column family, that features High Strength Silica (HSS) T3 particle technology for excellent retention of polar analytes with high efficiency and low pH stability.
Boost retention of polar acidic compounds by 50% while eliminating unwanted analyte-surface interactions using Atlantis BEH C18 AX Premier Columns, designed to achieve superior polar compound retention.
Optimize your laboratory’s productivity while addressing your budget realities with Waters Global Services. Maintain peak system performance, minimize down time, address scientific application challenges, and support stringent compliance requirements.
Maximize your lab resources and minimize risk with payment options from Waters Capital, which includes innovative solutions to upgrade aging instruments, customized support, and flexible options to bundle your complete laboratory solution in one easy monthly payment – everything you need to advance your science.
With the Waters FlexUP Technology Trade-In Program, you can cost-effectively refresh your aging instruments from Waters or another manufacturer — so your lab can work with the latest technology for optimum productivity and security.
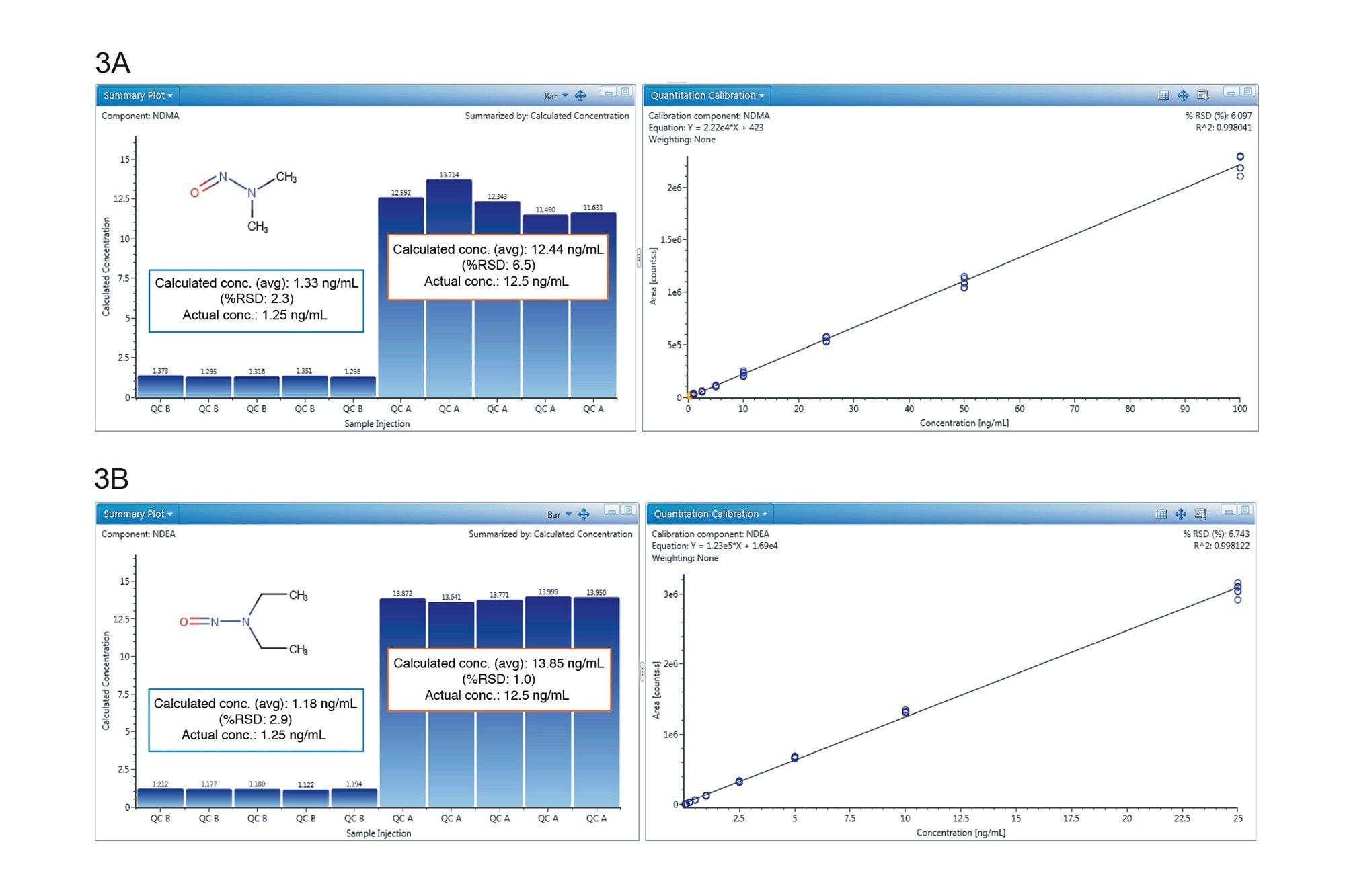
3A. NDMA summary plot of calculated concentrations (mean accuracy) at QC level A (12.5 ng/mL spike) and B (1.25 ng/mL spike), and calibration curve (0.1–100 ng/mL).
3B. NDEA summary plot of calculated concentrations (mean accuracy) at QC level A (12.5 ng/mL spike) and B (1.25 ng/mL spike), and calibration curve (0.025–25 ng/mL).