
A generic extraction and LC-MS/MS method, valid for a wide range of compound classes in a representative set of matrix types, was validated and shown to be suitable for the screening of 81 pesticide residue compounds in fruit and vegetables.
There is currently a wide range of pesticides that may be applied to agricultural crops in order to control undesirable weeds, insects, mites and moulds. Over 800 such compounds exist and most countries have regulations governing their use. Produce that is to be used for human consumption must contain less than the statutory Maximum Residue Level (MRL) of a given pesticide. It is therefore necessary to monitor fruit, vegetable and cereal crops for the presence of these residues and, in order to maximize the efficient use of valuable analytical resources; it is desirable to test for as many compounds as possible during a single analysis.
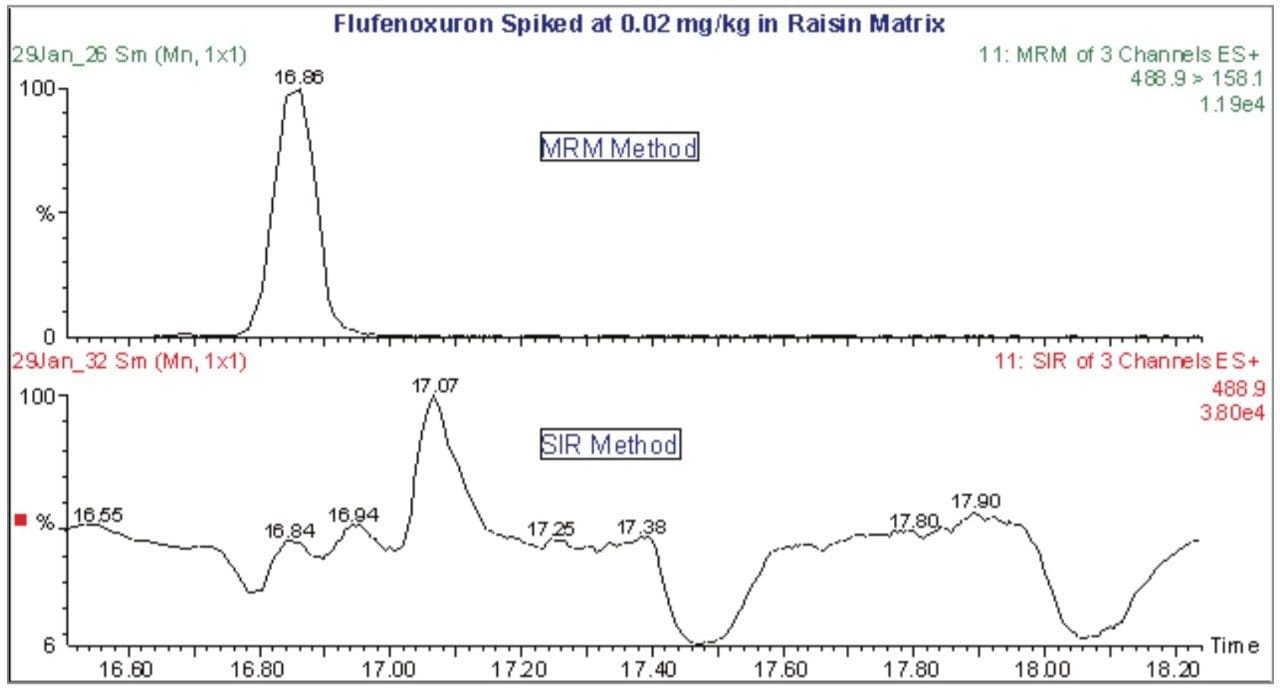
Multi-residue methods are normally used to target members of a single class of compound. This enables any extraction and cleanup process to be optimized for a particular type of chemistry. The extraction and cleanup of a range of different compound types is less selective and results in a more complex extract, increasing the potential for matrix interference during the determinative step. Triple quadrupole mass spectrometry in the Multiple Reaction Monitoring (MRM) mode provides the analytical selectivity required for achieving low analyte detection levels in a complex sample matrix. Figure 1 shows the difference in selectivity between an MRM and a Single Ion Recording (SIR) type of experiment when analyzing flufenoxuron at a spiked concentration of 0.02 mg/kg in raisin matrix. The compound is poorly distinguished from the interfering noise when monitoring the ion at m/z 488.9 in an SIR experiment, whereas it can be clearly detected by monitoring the collision induced dissociation of m/z 488.9 to m/z 158.1 in an MRM analysis.

Using the MRM technique a method was developed for the quantification of 81 pesticides and pesticide metabolites. A generic extraction and cleanup was performed. The method was validated for 5 commodities: - raisin, avocado, tomato, wheat flour, and lemon. These commodities represent high sugar (raisin), high fat (avocado) and high water (tomato), dry (wheat flour) and low pH (lemon) matrices. Target analytes include a number of compound classes such as carbamates, organophosphorous compounds, oximes and sulfonylureas.
The test sample is chopped avoiding loss of juice. An aliquot of 10 g is transferred into a blender cup. For dry sample materials like cereal grains, instant infant food or flour a homogenized portion of 5 g is weighed into the cup. Water is added to all samples to obtain 10 mL as a sum of natural and added water. To 10 g tomato (water content 95%), lemon (water content 90%) or avocado (water content 70%) 0.5 mL, 1 mL, and 3 mL of water are added respectively. To 5 g of raisins (water content 20%) and wheat flour (water content 10%) 9 mL and 9.5 mL of water is added respectively. In the case of dry sample materials it is necessary to wait 10 min after the addition of water. After a further addition of 20 mL methanol the sample is blended for 2 min. The total volume of supernatant extract (taking into account the natural water content of the sample) is 30 mL. In the case of very turbid extracts an aliquot is centrifuged at about 3000 g.
6 mL of the extract is mixed with 2 mL of a solution of sodium chloride (20 g in 100 mL water). An aliquot of 5 mL (which contains the pesticides residues of 1,25 g normal or 0,625 g dry sample material, respectively) is transferred to an extraction cartridge containing 5 mL of diatomaceous earth.
After a 5 min waiting period the cartridge is eluted with 16 mL of dichloromethane. The solvent of the collected eluate is gently evaporated. The dry residue is redissolved in 250 μL methanol with the help of an ultrasonic bath and further diluted with 1000 μL water. The resulting final extract contains the residues of 1 g normal or 0.5 g dry sample per millilitre. It is filtered through a 0.451 μm filter into a glass sample vial.
|
HPLC: |
Waters Alliance 2795 Separations Module |
|
Mobile phase A: |
MeOH/H2O (1:4 v/v) + 5mM CH3CO2NH4 |
|
Mobile phase B: |
MeOH/H2O (9:1 v/v) + 5mM CH3CO2NH4 |
|
Column: |
Waters Atlantis C18 4.6 mm id 100 mm with 3 mm particle size |
|
Flow: |
1.0 mL/min |
|
Injection volume: |
20 mL |
|
Approx 2:1 split of eluent before MS source |
|
Time 0 |
0% B |
|
Time 15 mins |
100% B |
|
Time 29 mins |
100% B |
|
Time 29.1 mins |
0% B |
|
Time 40 mins |
0% B |
A Waters Micromass Quattro micro API triple quadrupole mass spectrometer was operated in the positive ion electrospray mode. Nitrogen gas, at a flow rate of 850 L/hr and a temperature of 450 °C, was used for spray desolvation. The source block was maintained at 120 °C and the electrospray capillary voltage was 0.6 kV.
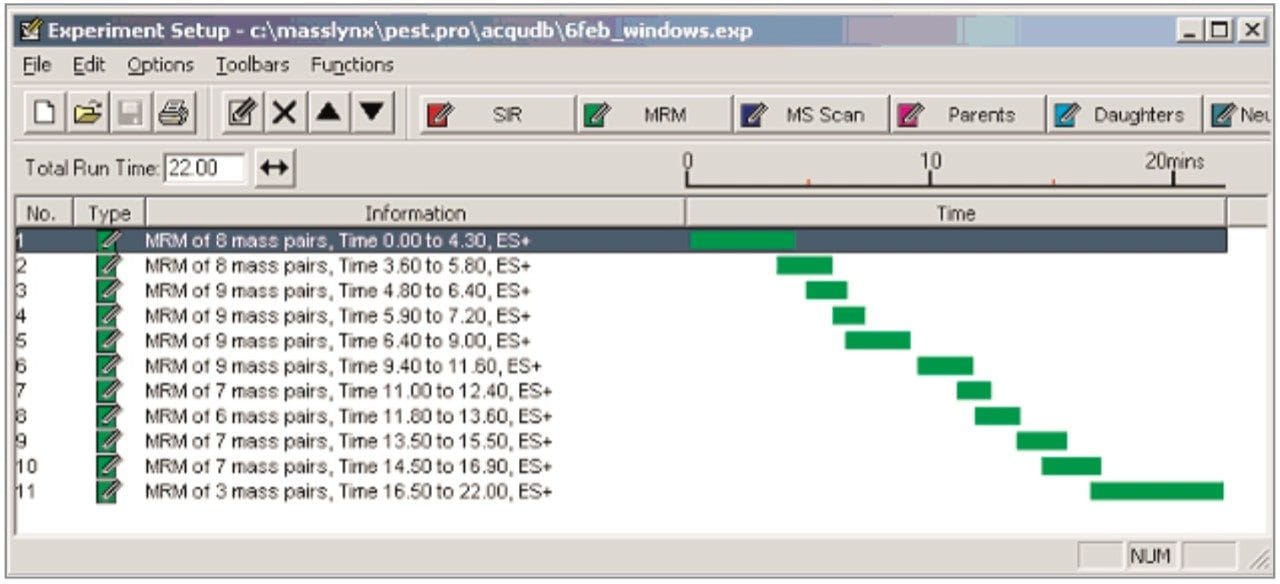
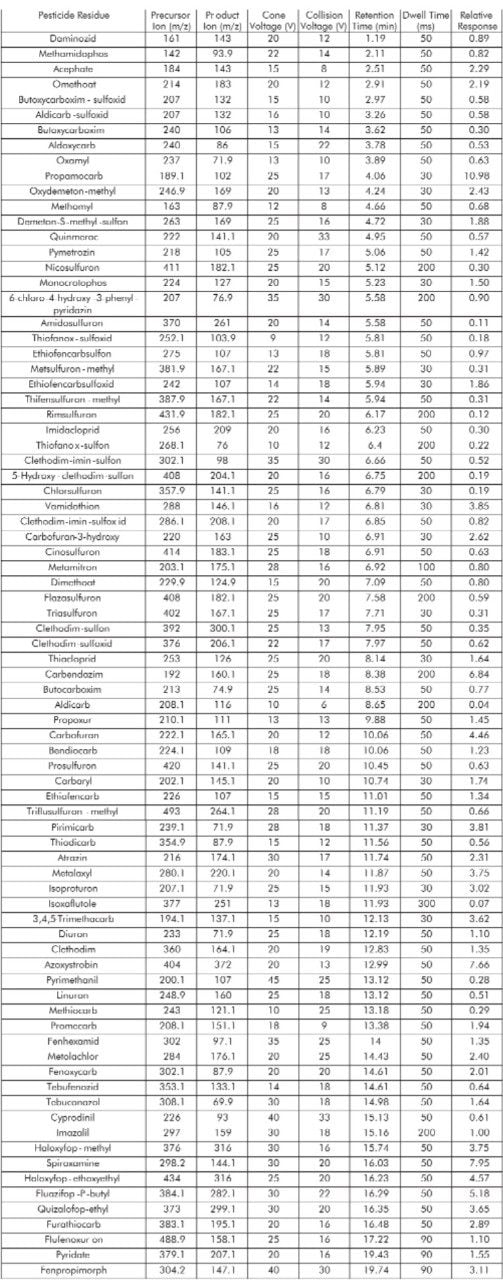
Figure 2 shows the distribution of MRM functions into windows based on analyte retention times. Such a system allows the flexible use of MRM dwell times, with less intense peaks having their S:N values increased by the use of longer dwell times whilst a short overall scan cycle time is maintained. For each pesticide residue the precursor and product ion m/z values, the cone and collision voltages and the retention and dwell times are given in Table 1. The relative response of each analyte, with reference to the response of Imazalil, is also shown. The relative response values were determined using the analysis of a solvent standard at the 50 pg/μL level.
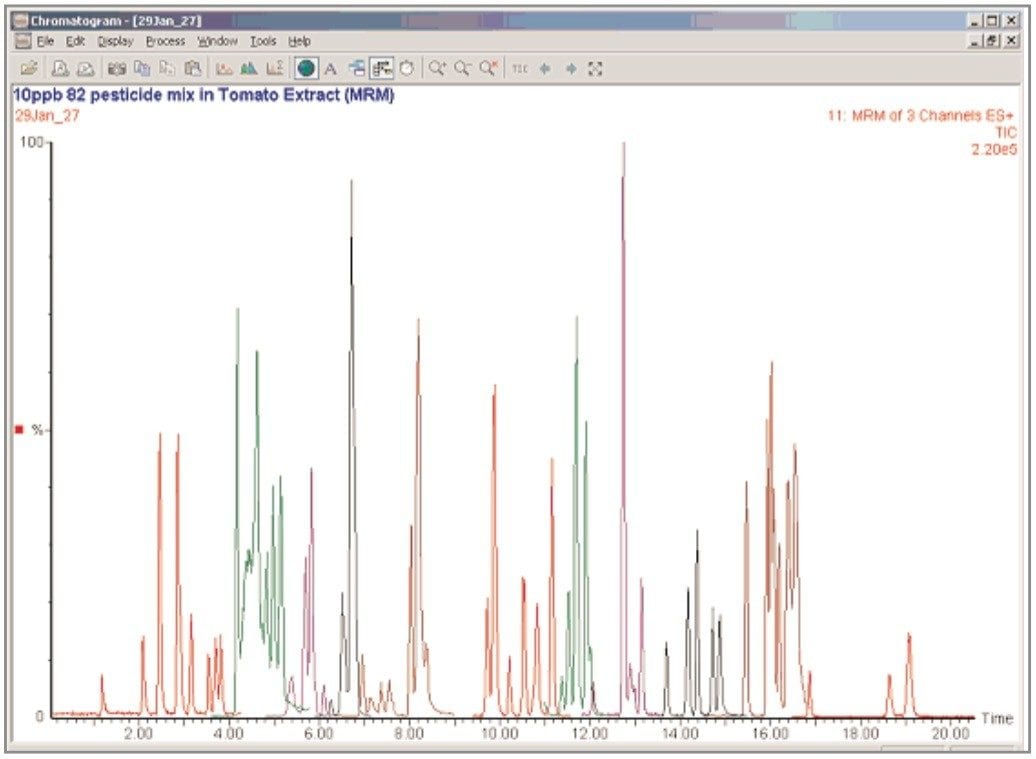
A chromatogram, showing the results from the analysis of a tomato extract spiked at 0.01 mg/kg, is shown in Figure 3.
For each of the five crop types, matrix-matched standards were generated at the 5, 20, 40, 60, 80, and 100 pg/μL levels for all analytes. These correspond to 0.005, 0.02, 0.04, 0.06, 0.08, and 0.1 mg/kg in tomato, avocado and lemon and to 0.01, 0.04, 0.08, 0.12, 0.16, and 0.2 mg/kg in raisin and wheat flour. Figures 4, 6, 8, 10, and 12 show representative calibration graphs for butocarboxim (Oxime Carbamate Insecticide) in tomato, pyrimethanil (Pyrimidine Fungicide) in raisin, promecarb (Phenyl Methylcarbamate Insecticide) in avocado, cyprodinil (Pyrimidine Fungicide) in wheat flour and vamidothion (Organothiophosphate Insecticide) in lemon respectively. Figures 5, 7, 9, 11, and 13 show chromatograms for these compounds at the lowest calibrated level of 0.005 mg/kg in tomato, lemon and avocado and 0.01 mg/kg in raisin and wheat flour.
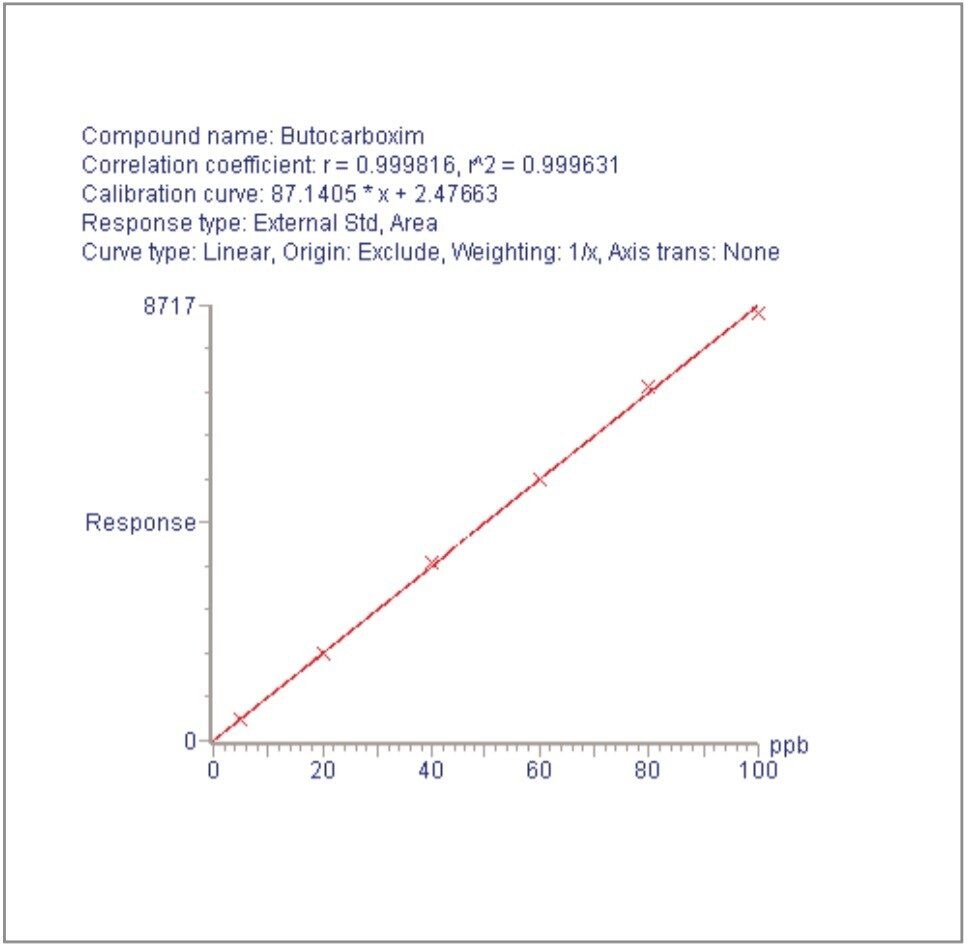
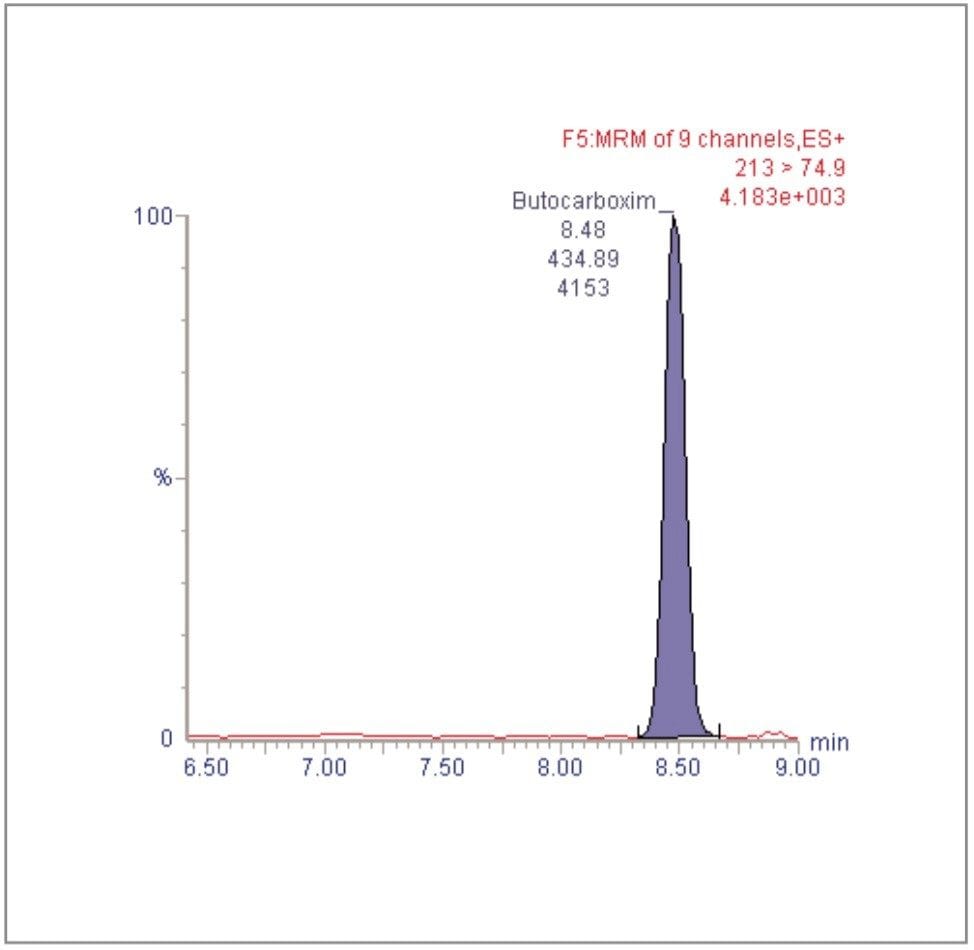
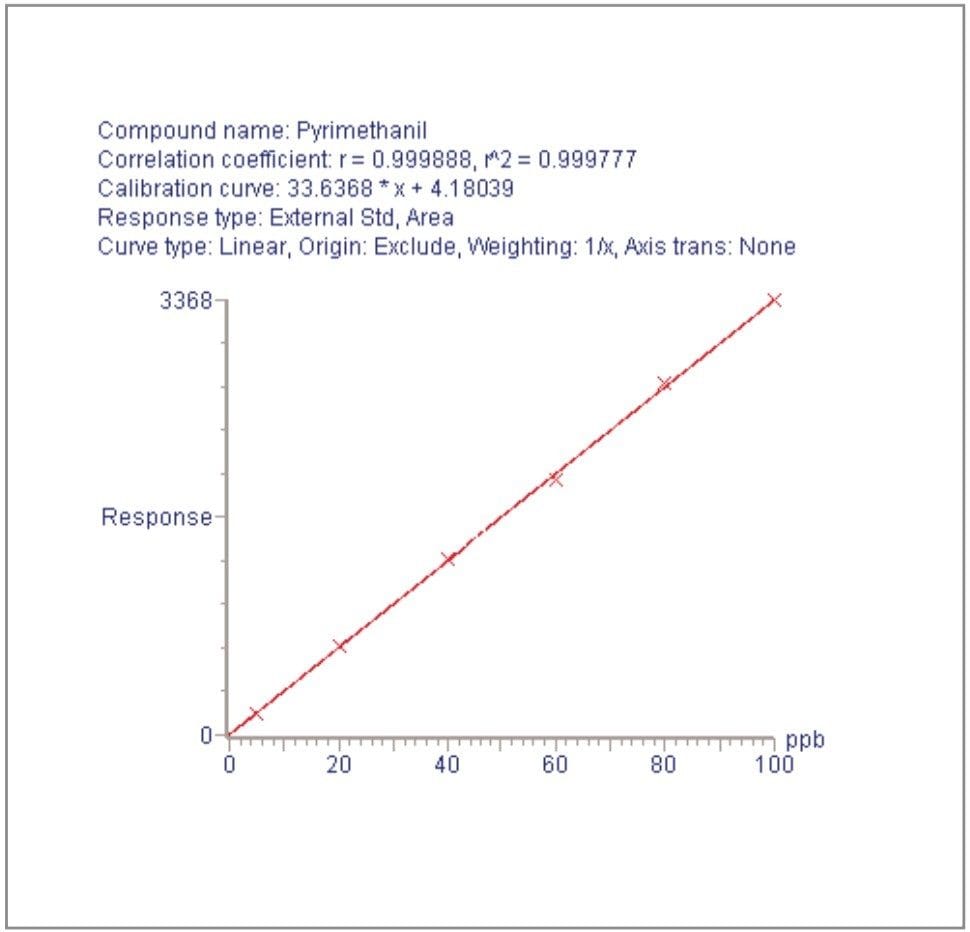
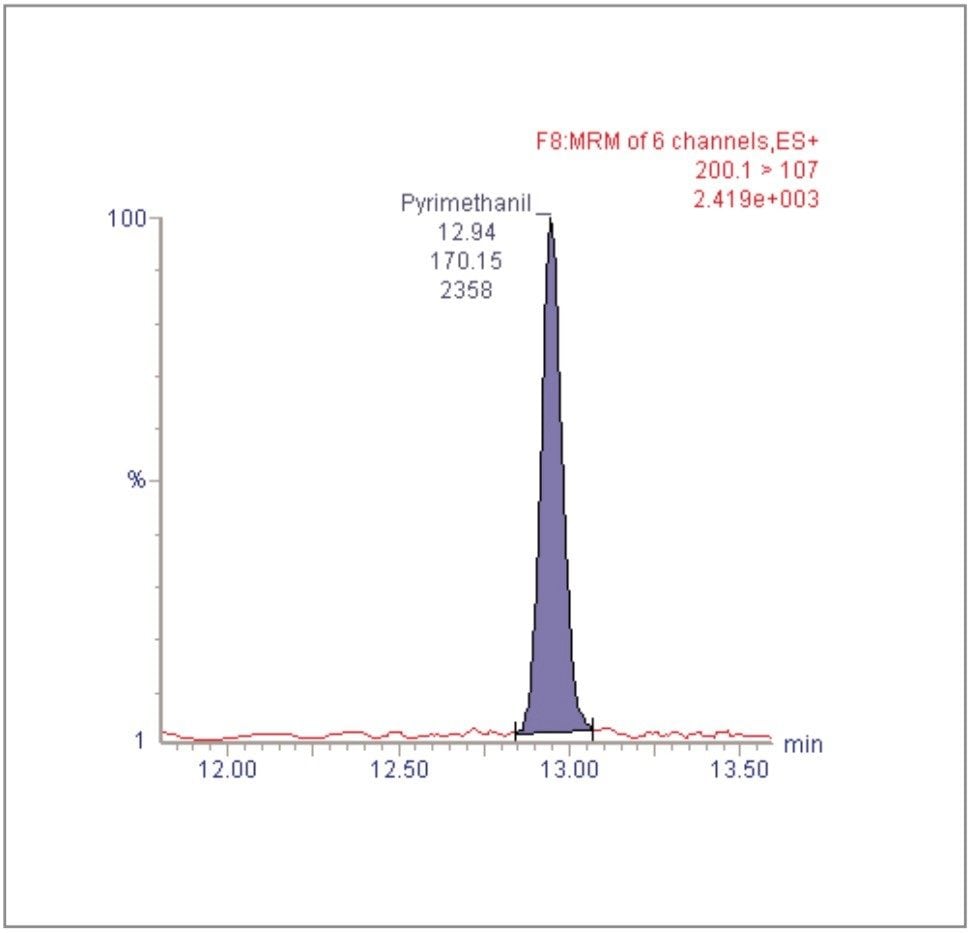
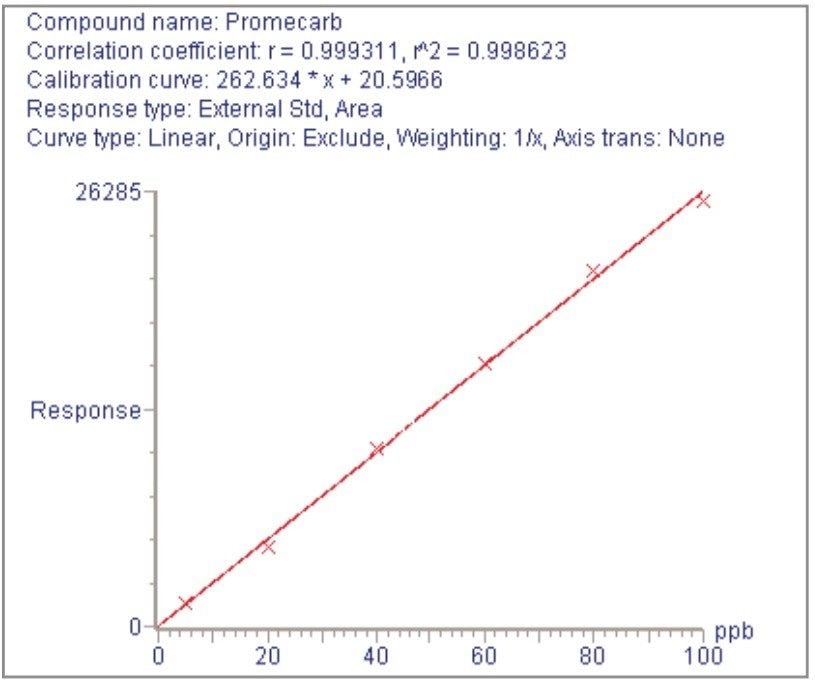
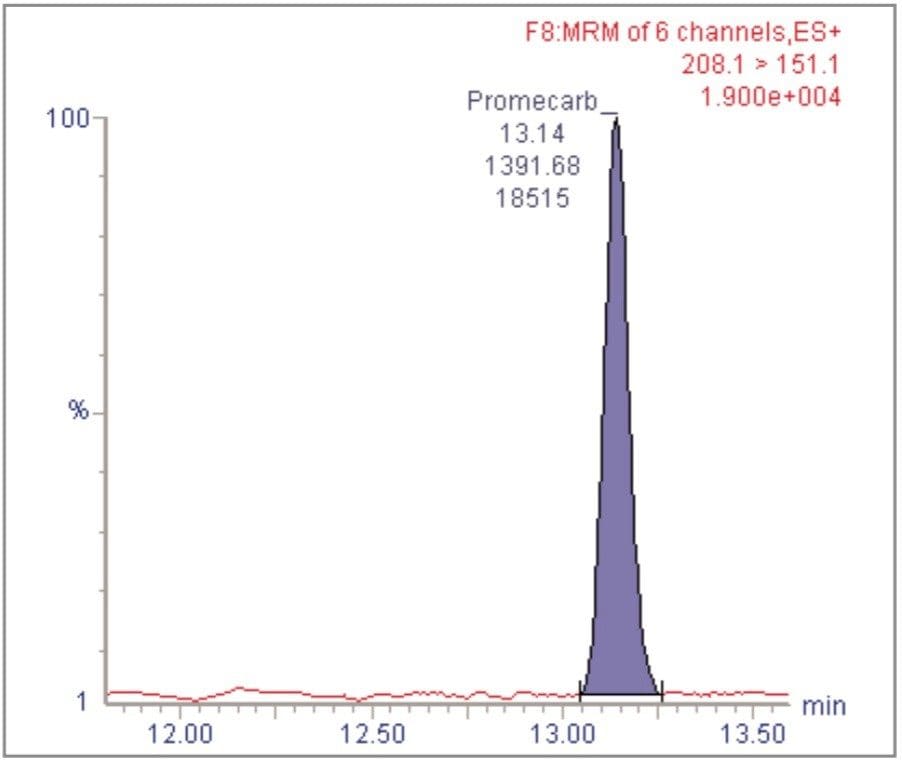
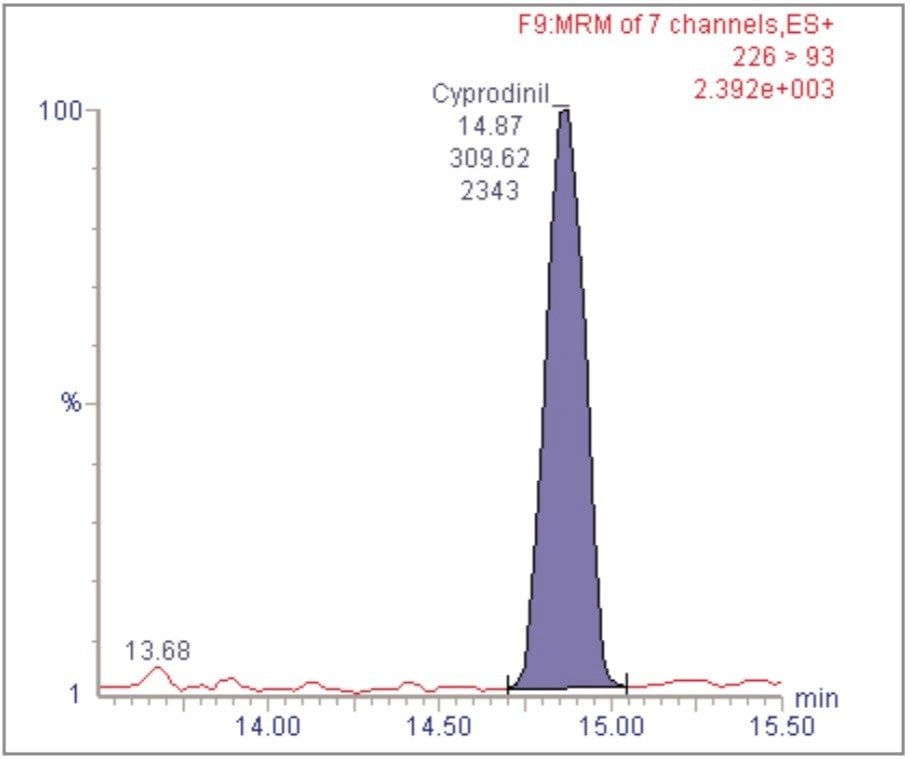
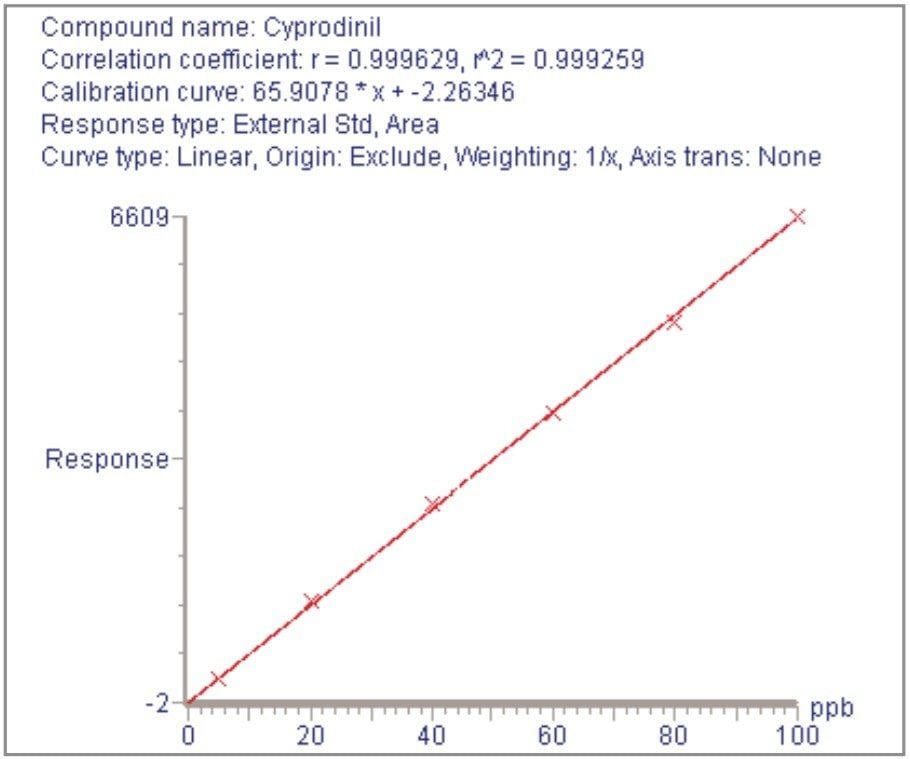
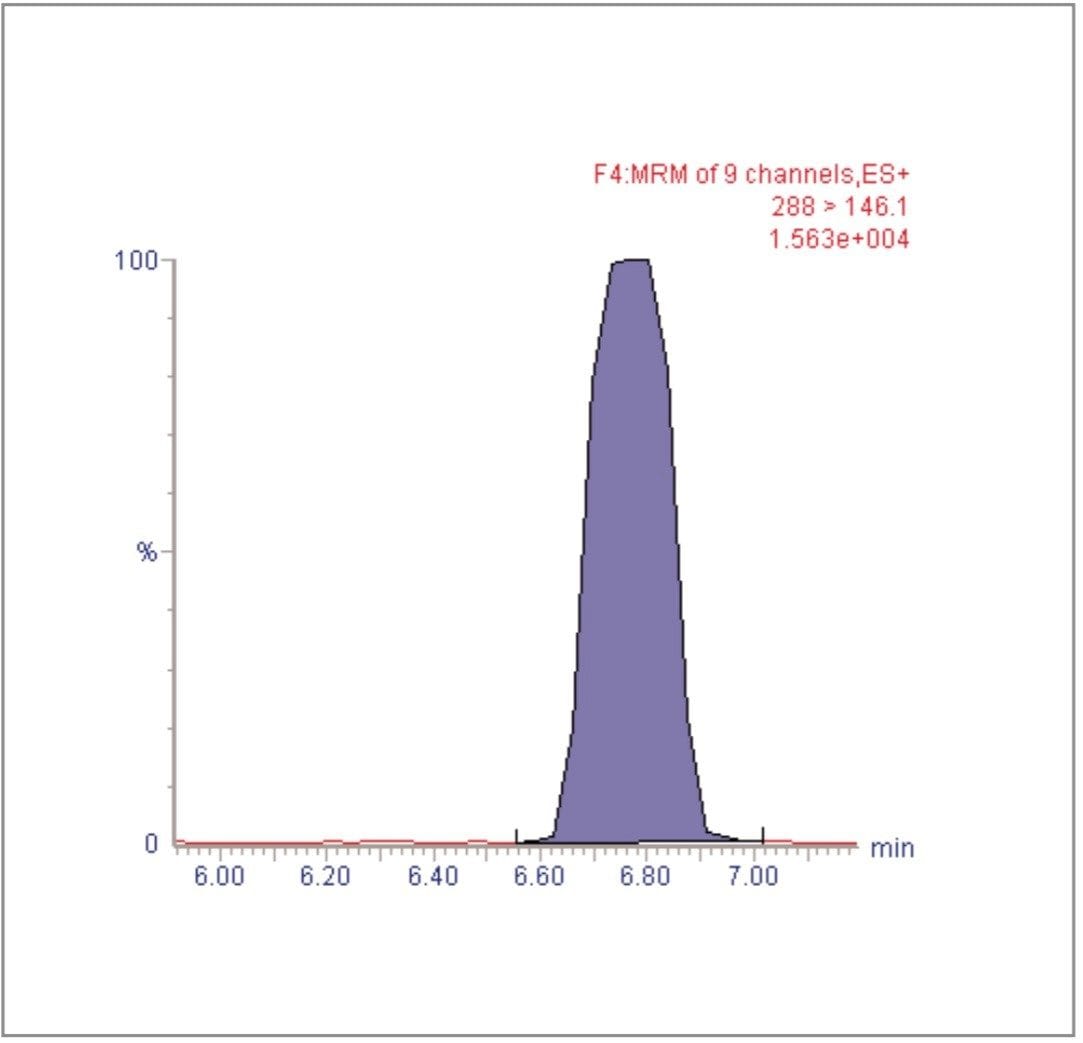
Five replicate analyses were performed on extracts, from each matrix type, spiked at levels equivalent to 0.01 and 0.05 mg/kg for tomato, lemon and avocado, and at 0.02 and 0.1 mg/kg for raisin and wheat. These analyses were interspersed between bracketing calibrations. Further application notes in this series, entitled "A Multi- Residue LC-MS/MS Method for the Determination of 81 Pesticide Residues in Fruit and Vegetables: Part 2…etc.", give details of method repeatability, at these levels, for each compound class. These application notes also give estimates of the Limits of Determination (LoD) for each compound.
A generic extraction and LC-MS/MS method, valid for a wide range of compound classes in a representative set of matrix types, was validated and shown to be suitable for the screening of 81 pesticide residue compounds in fruit and vegetables. The limits of determination achieved for the pesticides analyzed are generally well below that required for surveillance monitoring in the EU. Therefore the method is clearly extendable to greater numbers of pesticide targets within the compound classes examined.
720000686, June 2003