
A number of diagnostic steps are taken to understand assay failure. Waters IntelliStart Software provides a simple and automated system check procedure to ensure that a Waters LC-MS system is operating properly.
Multiple pressures exist for any laboratory, be it a need for fast turnaround of samples, to produce diagnostics for further tests or for purely economic or other reasons; but in all case maximum uptime is of paramount importance.
The highly-regulated bioanalytical laboratory must perform assays according to pre-designated criteria that are defined by validated methods. Primarily these are:
Routine assays that fail due to results that fall outside of the validation criteria impact the laboratory, not only in terms of having to re-run samples or even through the loss of samples; but also in the time taken to try to understand what has caused the assay to fail. If the failure of the assay is not recognized in a timely fashion, recovery of the assay and samples can have wide-ranging consequences for the trial.
Commonly, a number of diagnostic steps are taken to understand assay failure. As assays involve increasingly complex instrumentation, this generally requires the skills of an experienced user in order to troubleshoot the assay by performing and evaluating system checks. Waters IntelliStart Software provides a simple and automated system check procedure to ensure that a Waters LC-MS system is operating properly.
Performing system suitability checks is necessary but often laborious, involving replicate injections of a known compound to check the system integrity using the following criteria:
Measurement of these criteria requires a robust inlet method. For LC-MS methods, the single ion recording (SIR) or multiple reaction monitoring (MRM) transition of the test sample must also be known.
Software tools that automatically check the status of an assay allow any analyst to be confident of the integrity of an assay at any time, and also enable them to quickly determine the likely cause of assay failure. The ability to apply predetermined criteria for pass/fail adds flexibility and can provide the following benefits:
A regular check of system performance also provides reassurance that results obtained from assays are unaffected by external or aberrant factors.
IntelliStart’s Automated System Check offers a simple solution for multi-user, fast-paced, LC-MS laboratory environments with walk-up operation for analysts with any expertise level. IntelliStart allows the user to incorporate the analysis of a series of QC samples. This enables the user to perform an automated system check that evaluates the status of the LC and MS instrumentation.
The analyses can be carried out at any predetermined interval as a timed event, or if desired, manually. The criteria can easily be determined and amended to suit a particular application.
With the Waters System QC protocol, users of any ability level with the instrumentation can verify that an LC-MS assay is performing to specification. To enable this, the protocol is supplied with a pre-selected sample, mobile phase, gradient conditions, ionization technique, and MS method. These parameters are included with the software; the user can simply select the appropriate settings to carry out a system check.
If preferred, an assay-relevant sample can be used to carry out the system check with user defined criteria to determine pass/fail. For example, the qualified sample is injected six times at a predefined concentration (three times is the minimum). The resulting data are then processed and reported with checks for sensitivity, and chromatographic performance in terms of accuracy and precision, as demonstrated in Figure 1.
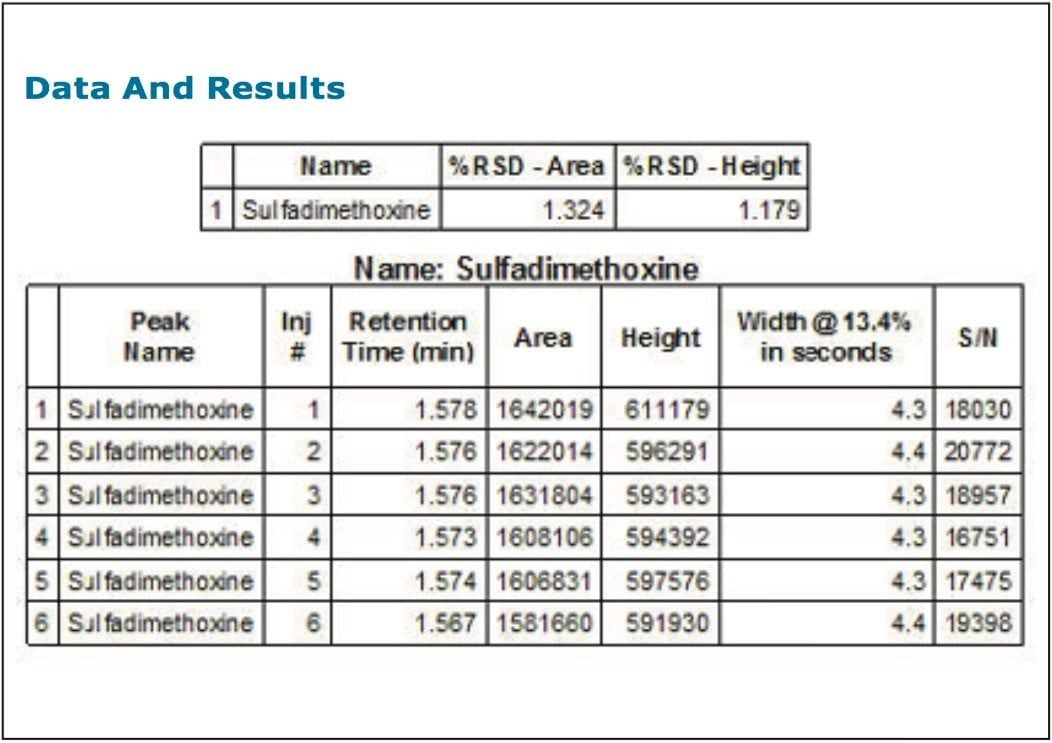
If the assay should fail, the raw data are then available to access accuracy, precision, or other parameters responsible for the out-of-specification result.
With the analyst’s ability to determine which parameters are relevant to the assay and conditions of the laboratory, this system suitability software is applicable across a wide range of conditions.
For full GLP compliance, the results of the system check, along with the raw data and experimental details, are automatically logged into the system. Reports can be produced in electronic or printed format.
720002620, May 2008