For forensic toxicology use only.
In this application note, we present a sensitive method for the quantification of morphine and its glucuronide metabolites. The method involves a simple SPE purification prior to analysis using LC/MRM and is suitable for plasma, whole blood or urine samples.
Morphine is a potent analgesic isolated from the opium poppy papaver somniferum and traditionally used for the treatment of moderate to severe pain. Analgesia results from the action of morphine at the opioid receptors of the spinal cord and brain (Figure 1), where it attenuates both the speed of the impulse and the perception of pain.
In human subjects, morphine is extensively metabolised (primarily by conjugation with glucuronic acid) to form morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G). Whilst, the principal metabolite i.e. M3G, has little or no analgesic effect, M6G has been shown to be highly effective and is believed likely to contribute significantly to the overall effectiveness of morphine.1 Hence, quantification of both the parent drug and metabolites is desirable for pharmacokinetic studies.
Previously we have described a LC-MS/MS method that allows the quantification of morphine and several other opiates in urine.2 Here we present a simple method that enables the quantification of morphine in plasma, whole blood and urine. Furthermore this procedure allows differentiation between two isobaric glucuronide metabolites.
Biological samples were prepared for LC-MS/MS analysis by means of a simple, solid-phase extraction (SPE) procedure. A Waters Oasis HLB extraction Cartridge (1 cc/30 mg) was firstly conditioned with 1 mL volumes of each of the following: methanol, water and ammonium carbonate (10 mM, pH 8.8). Samples (100 μL, spiked with deuterated internal standards) were made up to a final volume of 1 mL with ammonium carbonate before applying to the pre-conditioned cartridge. The cartridge was then washed with 1 mL ammonium carbonate before elution of the sample using 100% methanol (0.5 mL). Eluents were dried using a Savant Speedvac Plus evaporator and then redissolved in 100 μL of mobile phase. Reconstituted samples were briefly vortex mixed before the analysis of 10 μL using LC in conjunction with multiple reaction monitoring (MRM).
A Waters Quattro micro triple quadrupole mass spectrometer fitted with ZSpray ion interface was used for all analyses. Ionisation was achieved using electrospray in the positive ionisation mode (ES+). Details of the MRM conditions are given in Table 1.
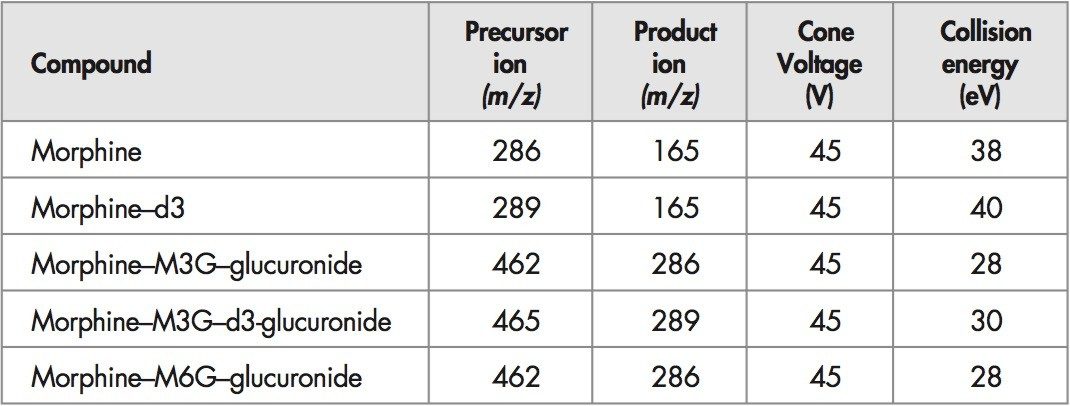
LC analyses were performed using a Waters 2795 separations module. Chromatography was achieved using a C18 column (3.9 x 150 mm) eluted isocratically with 0.1% formic acid:acetonitrile (97:3) at a flow rate of 0.3 mL/min. Column temperature was maintained at 30 ˚C. All aspects of system operation and data acquisition were controlled using MassLynx 4.0 software with automated data processing using the QuanLynx program.
A series of calibrators (0.5-500 μg/L) were prepared in duplicate by adding standards to blank plasma, whole blood or urine. Samples were then extracted using the SPE method described above prior to LC/MRM analysis.
Following LC/MRM analysis, the areas under the specific MRM chromatograms were integrated.
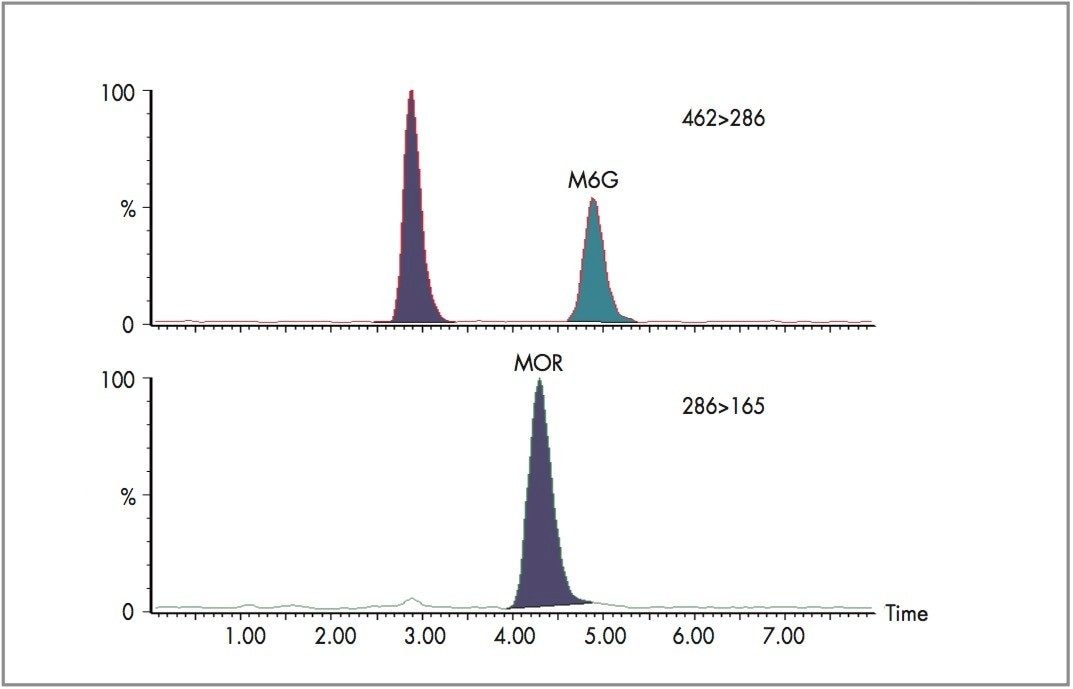
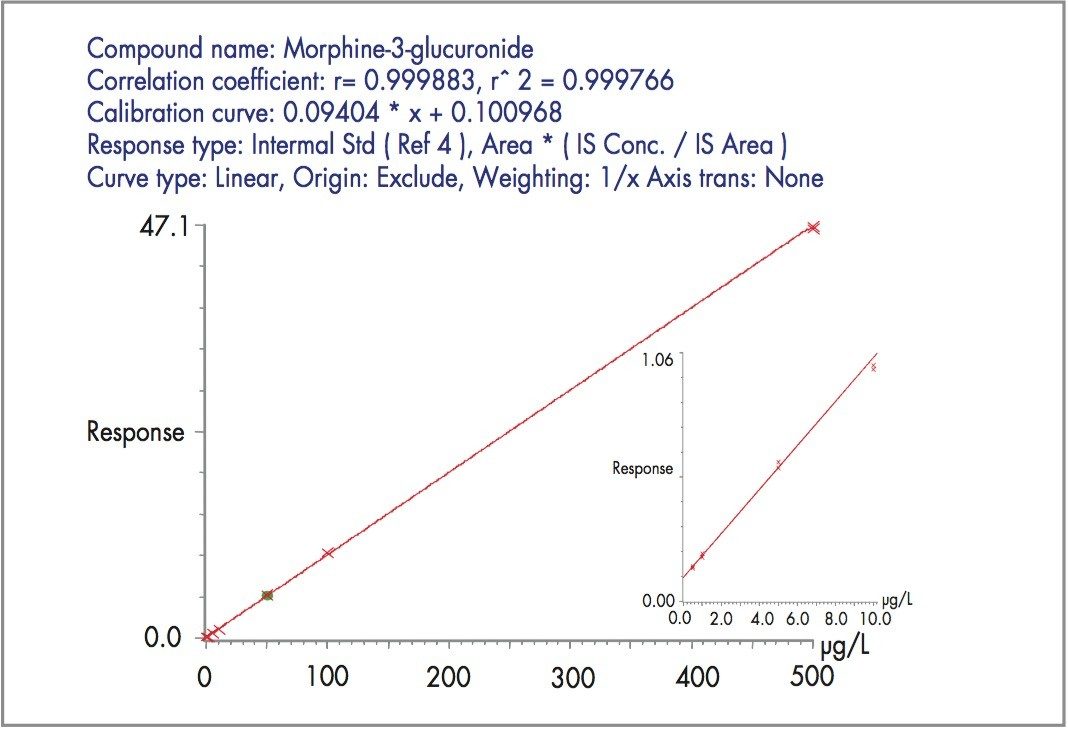
Figure 2 shows the extracted MRM chromatogram of morphine, M3G and M6G obtained with a 10 μL injection of the 5 μg/L plasma calibrator. Opiates were quantified by reference to the integrated area of the deuterated internal standards. Responses were linear (r = >0.999) over the range investigated for all 3 compounds and in each matrix (Figure 3 shows a typical standard curve for M3G in urine).
We present a sensitive method for the quantification of morphine and its glucuronide metabolites. The method involves a simple SPE purification prior to analysis using LC/MRM and is suitable for plasma, whole blood or urine samples.
720001546, March 2007