
This application note describes a 10 minute separation and identification method for analysis of edible oils with Waters ACQUITY UPLC/PDA System using low toxic solvents, acetonitrile, and 2-propanol as the mobile phase.
Seed oil is an important component of food, fuel, soap, cosmetic, and personal care products. Approximately 100 million tons of oil are commercially produced worldwide from 22 different plants. The largest volumes are from soybean, peanut, olive, sunflower-seed, safflower, cotton-seed, rapeseed, and mustard.1 Oil quality and suitability for human consumption are very dependent on the source and quality of the raw materials, extraction methods, process conditions, product transportation, and storage conditions.2–5 Both the origin of the source plant and production quality are reflected in the price of the final seed oil product. Since the worldwide structure of edible oil production, marketing and distribution is fragmented, it is important to analyze oil products for their origin and purity in order to satisfy legislation and public health concerns.5–8
Triglycerides (TAG) are the major components of edible oils. Each seed oil possesses a unique composition of TAG, which can be used to help identify the geographic origin and detect adulteration.5,9–10 TAG can be indirectly analyzed through transesterification to fatty acid methyl esters (FAME), which then are analyzed using GC.5 The transesterification of TAG is time-consuming and not always quantitative. Non-aqueous reversed-phase liquid chromatography and silver-ion chromatography methods can be used to directly separate and characterize TAG.9–13 Most of these methods require a long run time (30 to 80 minutes) and use halogenated solvents in the mobile phases. However, the halogenated solvents are known carcinogens, restricted, and sometimes prohibited in laboratories.
This application note describes a 10 minute separation and identification method for analysis of edible oils with Waters ACQUITY UPLC/PDA System using low toxic solvents, acetonitrile and 2-propanol as the mobile phase. The UPLC/PDA method not only allows faster and more precise analysis of TAG to authenticate the edible oils but also detects oxidized and decomposed TAG, enabling simultaneous determination of oil purity. The ability to quickly and unambiguously analyze the purity of seed oils can improve and accelerate the quality control of raw materials and finished products in food, cosmetic, and personal care industries. The sensitive testing method can be used to screen for oil quality and adulteration to protect consumer and public health worldwide.
Edible oils were purchased from local supermarkets and a chemical reagent vendor (SA). They were randomly labeled as brand H, P, SA, and SS. The oils were diluted with 2-propanol to make 1 and 2 mg/mL solution for UPLC analysis. The standards, glyceryl trilinoleate (TAG_3C18:2), and Glyceryl trioleate (TAG_3C18:1) were diluted with 2-propanol to make 0.5 mg/mL solution for analysis.
|
System: |
ACQUITY UPLC/ACQUITY PDA |
|
Software: |
Waters Empower 2 |
|
Detection: |
PDA 195 to 300 nm |
|
Sampling rate: |
20 pts/s |
|
Filter response: |
fast |
|
Weak wash: |
2-propanol (600 μL) |
|
Strong wash: |
2-propanol (600 μL) |
|
Seal wash: |
90:10 Water: CH3CN (5 min) |
|
Column temp: |
30 °C |
|
Injection: |
2 μL (full loop) |
|
Mobile phase A: |
CH3CN (Fisher, Optima) |
|
Mobile phase B: |
2-propanol (Fisher, Optima) |
|
Column: |
ACQUITY UPLC 1.7 μm BEH C18 2.1x 100 mm |
|
Flow rate: |
0.28 mL/min |
|
Linear gradient: |
10% to 90%B in 10 minutes |
|
Column: |
ACQUITY UPLC 1.7 μm BEH C18 2.1x 150 mm |
|
Flow rate: |
0.15 mL/min |
|
Linear gradient: |
10% to 90% B in 22 minutes |
The ACQUITY UPLC high-pressure fluidic modules enable the analysis of seed oils with the ACQUITY small particle (1.7 μm) column technology using a UV-detector compatible mobile phase, acetonitrile and 2-propanol, to give fast, sensitive and high resolution separations. TAG components of edible oils can be detected at 210 nm wavelength and a 10 minute linear gradient method separates the edible oil components. Figure 1 shows PDA extracted 210 nm chromatograms of soybean, olive, canola, peanut, and corn oils as well as two TAG standards using a 2.1 x 100 mm BEH C18 Column.
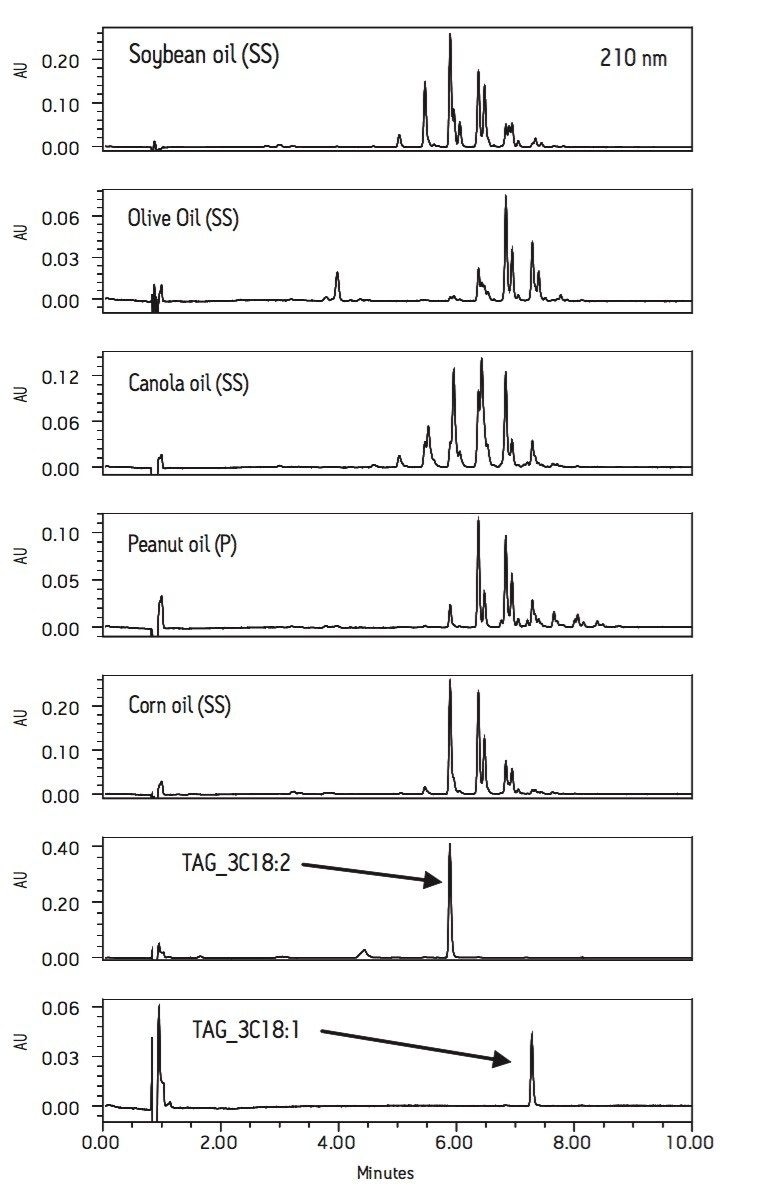
Comparison shows that each oil has a distinct chromatographic pattern. This confirms that UPLC can be used to rapidly determine the type of oil and precisely detect adulteration.14
The observed UPLC peak patterns of the seed oils are similar to the reported results of non-aqueous reversed-phase HPLC.9,10 However, the UPLC separation shows higher resolution and is achieved in less than one-third of the run-time relative to the HPLC methods. The retention times of TAG_3C18:2 and TAG_3C18:1 confirmed that the separation of TAG was based on the chain length of fatty acid and the total number of double bonds.9,10 TAG with shorter chain length and higher number of unsaturated bonds elutes earlier. The mobile phases used in the current experiments are highly compatible with mass spectrometry detectors, if needed to obtain additional structural information.
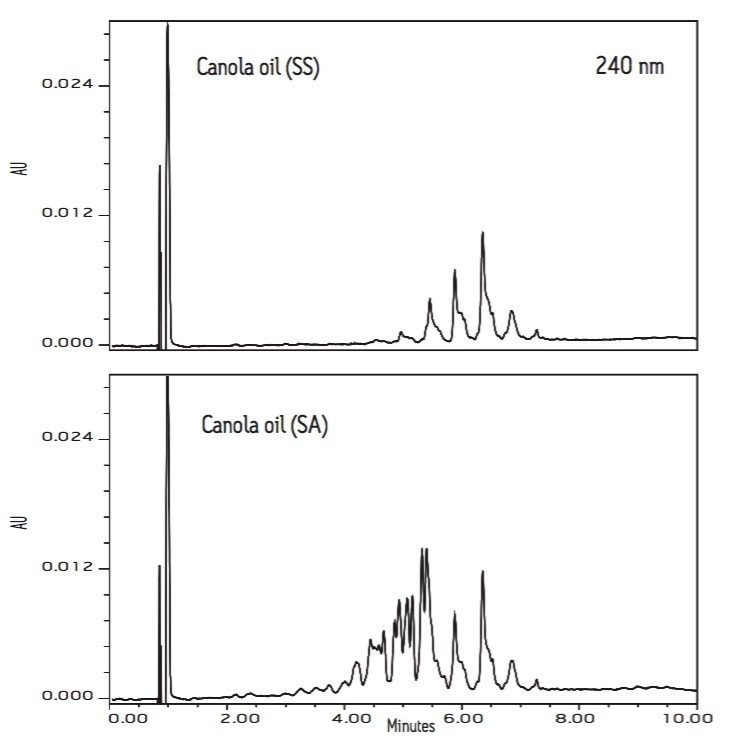
Figures 2 and 3 compare the chromatograms (210 nm) of three soybean oils (SS, H, and SA) and two canola oils (SS and SA).
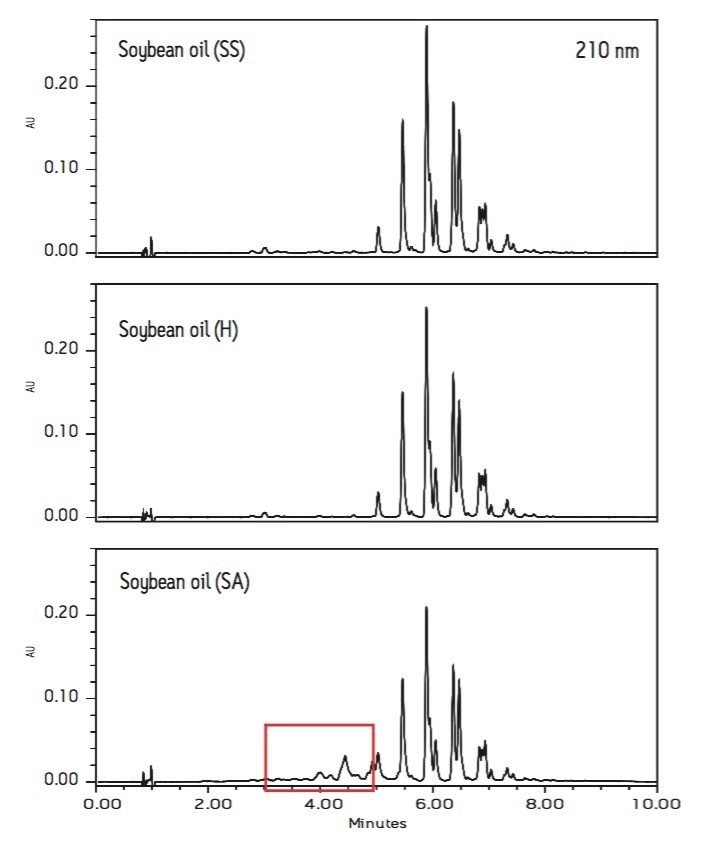
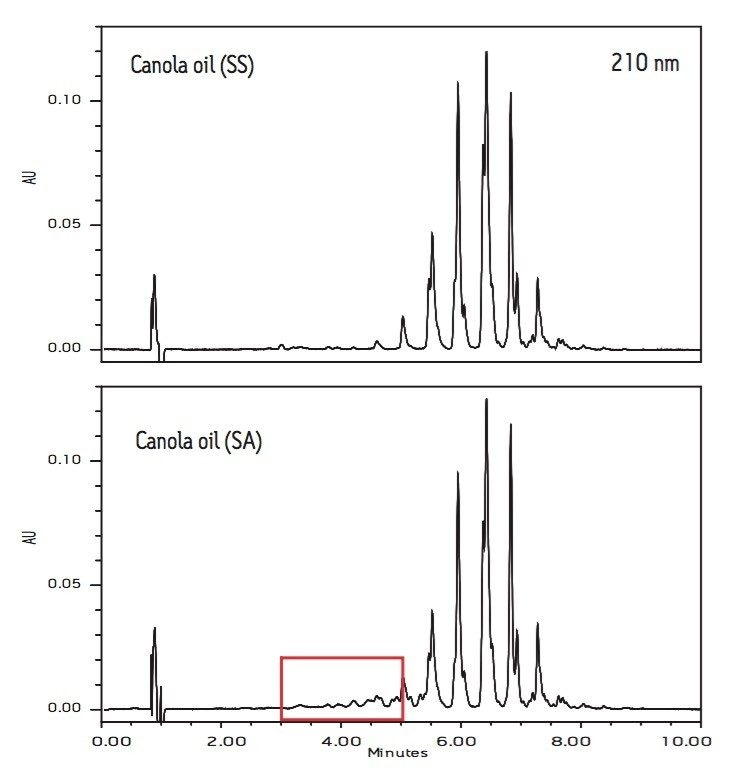
The soybean oils from vendors SS and H have identical chromatographic patterns. However, the chromatogram of soybean oil (SA) shows many additional peaks with retention times between 3 to 5 minutes. It is worth noting that the chromatogram of canola oil from vendor SA also has more peaks than the chromatogram of canola oil (SS) in the same retention time range. These extra peaks are indicators of lower quality.
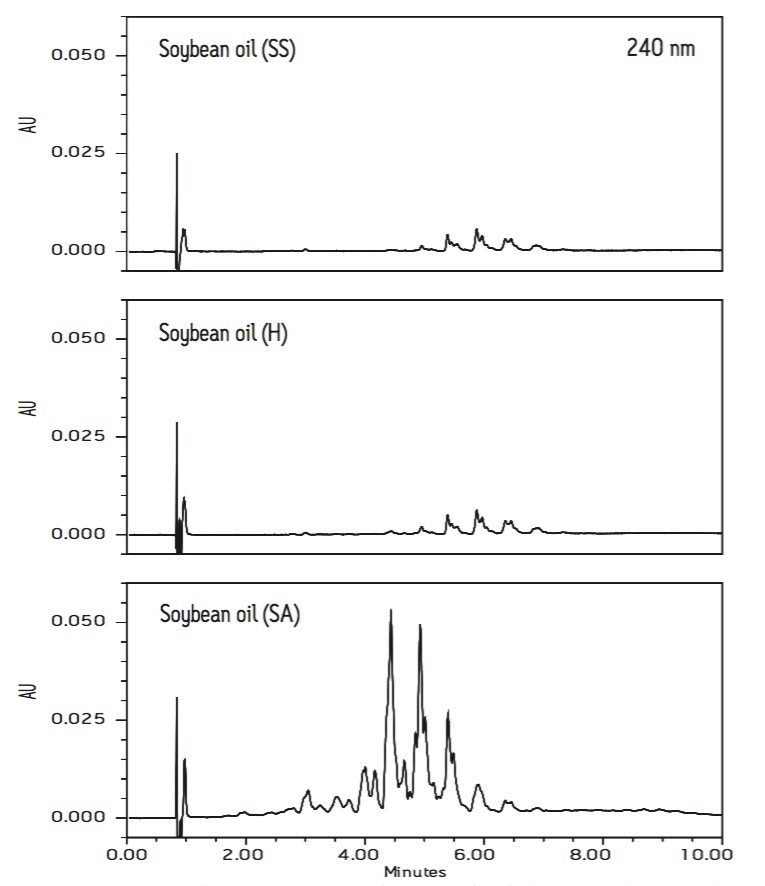
Edible oils are subject to oxidation and eventually decompose, turning rancid. The hydroperoxides (oxidized TAG) and decomposed TAG (fatty acid with three conjugated double bonds) have UV absorption at 240 nm wavelength. Figures 4 and 5 show the PDA extracted 240 nm chromatograms of three soybean oils (SS, H, and SA) and two canola oils (SS and SA), respectively. Again, the chromatograms of both soybean and canola oils from vendor SA have many additional peaks with retention times between 3 to 5 minutes. The increased peak response at 240 nm shows that the oil contains more oxidized and decomposed TAG components, indicating poor oil quality. This shows the added value of the UPLC method rapidly evaluating the quality and impurity of edible oils.
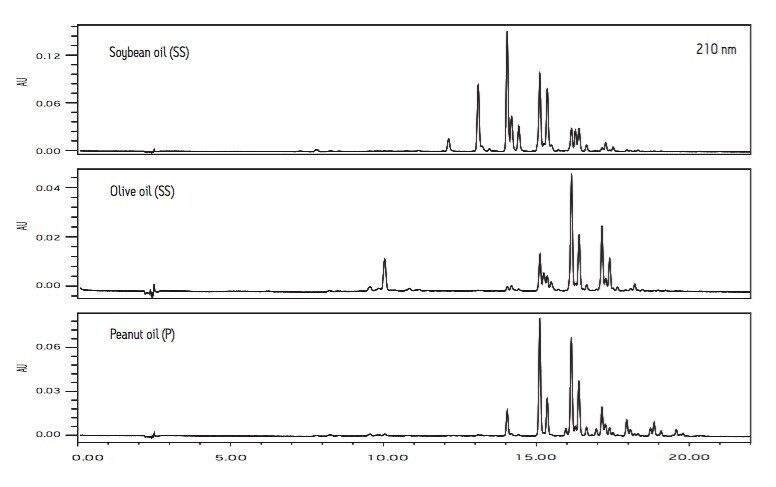
Taking advantage of the high-pressure fluidic modules of UPLC system, a longer BEH C18 column (2.1 x 150mm) was used to further separate the TAG components of edible oils. Figure 6 shows the PDA extracted 210 nm chromatograms of soybean, olive, and peanut oils. The data show that many previously unresolved TAG pairs are now separated. This higher resolution UPLC method can be further exploited, perhaps, to better identify the geographic origin of a particular seed oil or to assess adulteration.
The Waters ACQUITY UPLC with PDA detector is an ideal system for the analysis of seed oils. It enables rapid, sensitive, high resolution separations, and can provide data for determining both origin and purity of oils in a single experiment. The separation is several times faster than conventional HPLC methods and does not use toxic halogenated solvents. The UPLC system not only provides fast and superior analytic solutions but also reduces solvent consumption and hazardous solvent waste, resulting in cost and safety benefits. Potential applications of the UPLC method include analyses of food, cosmetic, personal care, bio-diesel, FFA (free fatty acids), FAME and TAG as well as screening edible oil for adulteration.14,15
http://www.unitedsoybean.org/soystats2001/page 39.htm
J.R. Morello et al. JAOCA. 2006, 83 (8): 683–690.
M.A. Brescia et al. JAOCA. 2003, 80 (10): 845–950.
M.Paz Romero et al. JAOCA. 2003, 80 (5): 423–430.
V.G. Dourtoglou et al. JAOCS. 2003, 3 (203): 203–208.
http://www.thehindubusinessline.com/2003/06/10/stories/2003061000361100.htm
http://www.dpi.nsw.gov.au/aboutus/news/recent-news/agriculture-news-releases/aussie-oil-true-blue
http://www.unctad.org/infocomm/anglais/olive/sitemap.htm
P. Sandra et al. J Chromatogr. 2002, A (974): 231–242.
V. M. Kapoulas et al. J Chromatogr. 1986, 386 (311–320).
C.A. Dorschel, JAOCS. 2002, 79 (8), 749–753.
M. Romeu-Nadal et al. Analytica Chimica Acta. 513, 457–461.
LCGC, The Application Notebook. 2006, Sept. 1: 51.
P. J. Lee, C. H. Phoebe, A.J. Di Gioia, “ACQUITY UPLC Analysis of Edible Oil (Part 2): Olive Oil Quality & Adulteration”, Waters Corporation, 2007: 720002026EN.
ACQUITY UPLC/ELS/UV One Methodology for FFA, FAME and TAG Analysis of Bio-diesel Fuels. Waters Corporation, 2007: 720002155EN.
720002025, April 2007