
Hydrophilic interaction chromatography (HILIC) is a chromatographic technique that has been used to improve the retention of very polar species that are poorly retained in reversed-phase HPLC (RP-HPLC).
Many polar compounds such as amino acids, oligonucleotides, and small molecules (i.e., metabolites) have important biological significance. Hydrophilic interaction chromatography (HILIC) is a chromatographic technique that has been used to improve the retention of very polar species that are poorly retained in reversed-phase HPLC (RP-HPLC). This is achieved by utilizing a high organic, low aqueous mobile phase in combination with a polar stationary phase. Using this chromatographic technique with highly efficient, 1.7 μm UPLC ethylene bridged hybrid (BEH) particles results in faster methods that exhibit improved polar retention, higher sensitivity, enhanced chromatographic resolution, and significantly improved column lifetime. Chromatographers can, therefore, meet the challenges of developing separations that completely characterize the constituents of samples.
HILIC offers several benefits over RP-HPLC with regards to MS response and simplification of sample preparation methods. Due to the highly organic (> 80%) mobile phase utilized in HILIC, signal intensity in electrospray MS is improved through efficient mobile phase desolvation and compound ionization. Additionally, sample clean-up by protein precipitation or SPE can be directly analyzed without solvent evaporation and reconstitution, reducing sample handling steps and increasing the number of samples that can be analyzed. In addition, 1.7 μm ACQUITY UPLC BEH HILIC Columns use the same BEH particle technology as XBridge HILIC HPLC Columns. Therefore, scalability between HPLC and UPLC is easily achieved.
All separations were performed on a Waters ACQUITY UPLC System. Detection was performed with either an ACQUITY UPLC PDA Detector, or with an ACQUITY TQD Mass Spectrometer. All other conditions (column dimensions, temperature, flow rate, mobile phases, etc.) are specified in the figure captions or text.
The target buffer concentration in the mobile phases was 10 mM for all experiments. First, a 200 mM concentrated stock buffer was prepared at each pH. The stock buffer was then diluted 20-fold with varying amounts of water and acetonitrile to achieve the desired mobile-phase composition. For example, 1 liter of 95/5 ACN/H2O mobile phase containing 10 mM ammonium acetate with 0.02% acetic acid was prepared by adding 50 mL of the concentrated stock buffer (200 mM ammonium acetate with 0.4% acetic acid) to 950 mL of ACN.
Mass spectrometry detection was performed using the ACQUITY TQD Mass Spectrometer operated in either selected ion recording (SIR) or multiple reaction monitoring (MRM) mode. All experiments were conducted using positive electrospray. Parameters including capillary voltage, cone voltage, desolvation and source temperature, and collision energy were optimized for each of the compounds analyzed. Dwell times were typically 25-50 ms with an interscan delay of 10 ms and an interchannel delay of 20 ms.
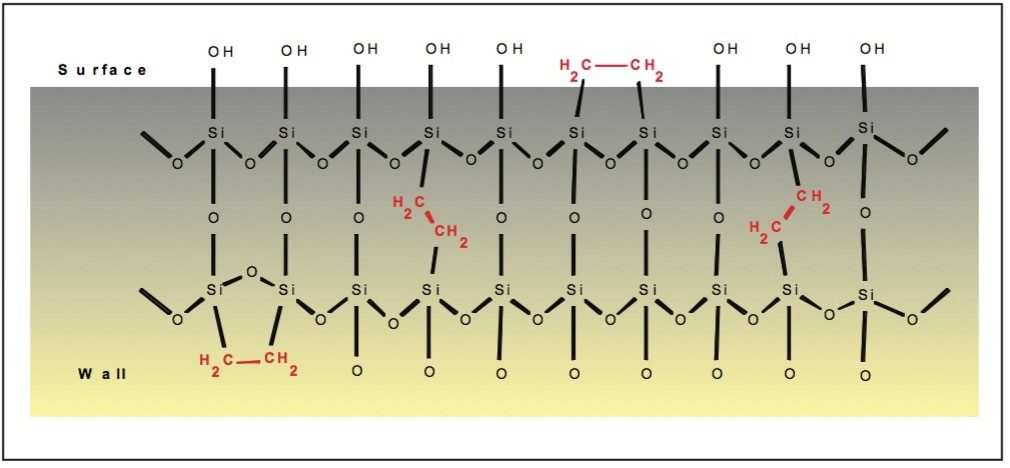
The structure of the BEH particle substrate is represented in Fig. 1. Since the bridging ethylene groups are embedded into the silica matrix, nearly one third of the surface silanols are removed.1 Thus, there is a reduction in secondary retention mechanisms on these surfaces compared to 100% silica substrates.
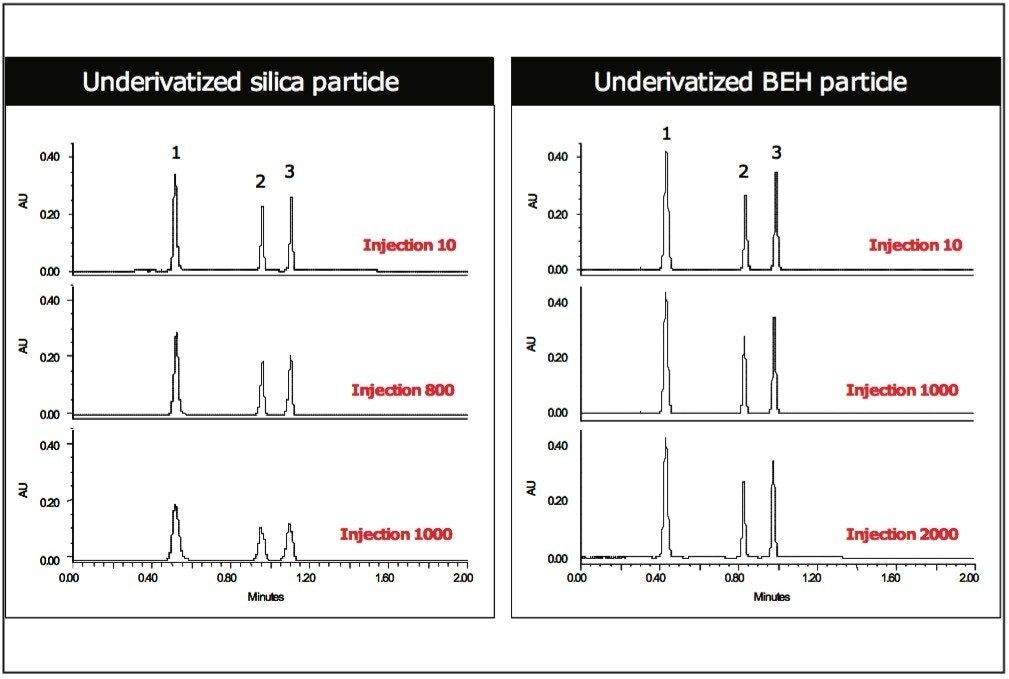
In addition to reduced secondary interactions with residual silanols, BEH particles also exhibit chemical stability superior to that of silica particles. This is illustrated in Fig. 2, where the stability of columns containing underivatized silica and BEH particles was compared under gradient conditions at pH 5.0 using a mixture of three polar compounds. The performance of the silica-based column deteriorated rapidly after ~ 800 injections, whereas the BEH Column showed no change in chromatographic performance in HILIC mode over the course of 2,000 injections.
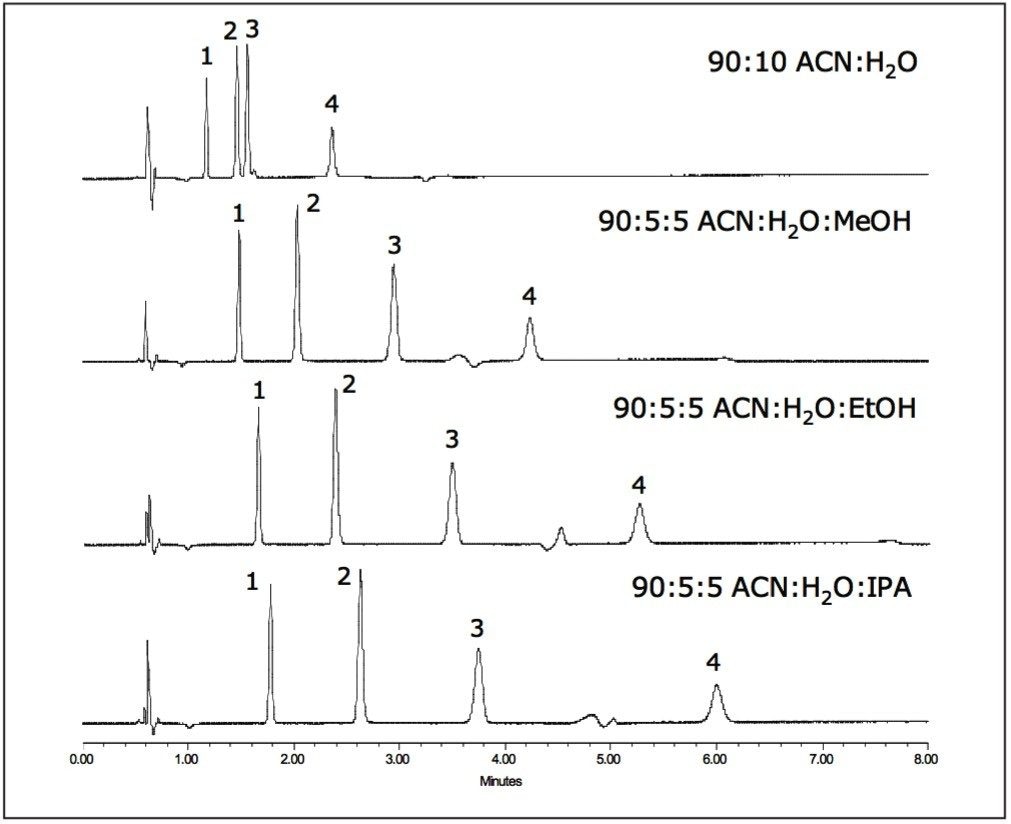
In order to maximize retention in HILIC, the aqueous content of the mobile phase must be reduced to as low as 2.5%.2,3 However, it is important that there is some water present in the mobile phase in order to coat the stationary phase sufficiently and to promote retention by enabling analyte partitioning into this water layer. Substituting some of the water in the mobile phase with other polar solvents (i.e., MeOH, EtOH, and IPA) can lead to improved retention of highly polar compounds (Fig. 3). In contrast to RP-HPLC, these polar solvents are weaker elution solvents than water in HILIC, and increased retention is observed with decreasing polarity. This may be useful when trying to develop and optimize retention and selectivity in HILIC separations.
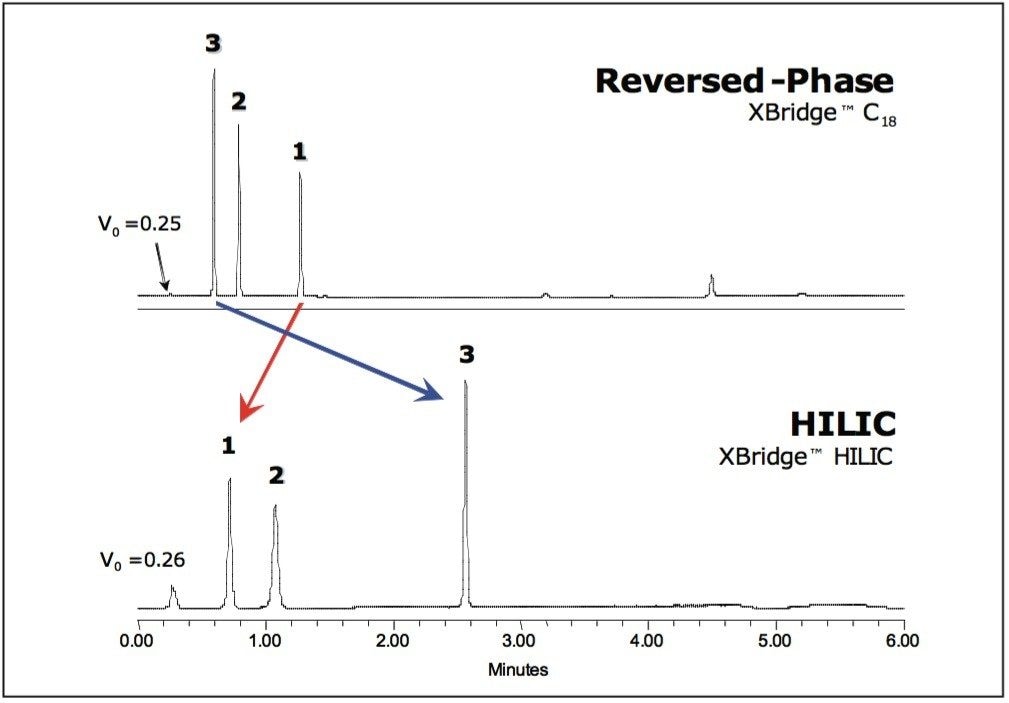
In RP-HPLC, polar compound retention is traditionally achieved using mobile phases that contain high percentages of water. Under these conditions, reversed-phase packings can experience pore dewetting, where water is driven out of the particle pores after flow through the column stops. The result is a change in the column void volume and a dramatic loss of analyte retention.4
Since HILIC separations are essentially performed in a mode opposite to that of RP separations, their selectivity is complementary (Fig. 4). This explains why HILIC is not only useful for retaining compounds that are not well retained in RP-HPLC, but is also beneficial for developing orthogonal separations for maximum peak capacity.
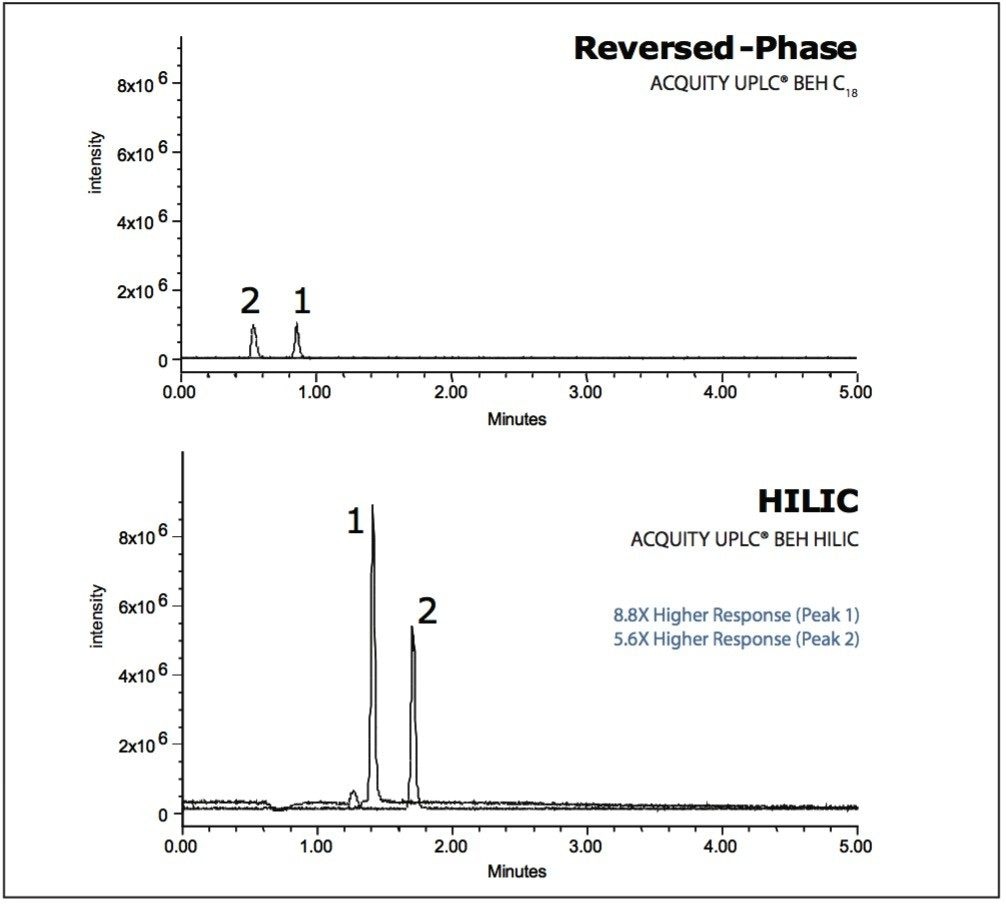
Adding ion-pairing reagents to the mobile phase is another way to achieve retention of polar compounds in RP-HPLC. However, these additives can suppress ionization efficiency in MS. Since HILIC mobile phases contain a high amount of organic solvent, desolvation efficiency is dramatically improved, and MS signal intensity is substantially higher than in RP-HPLC (Fig. 5).
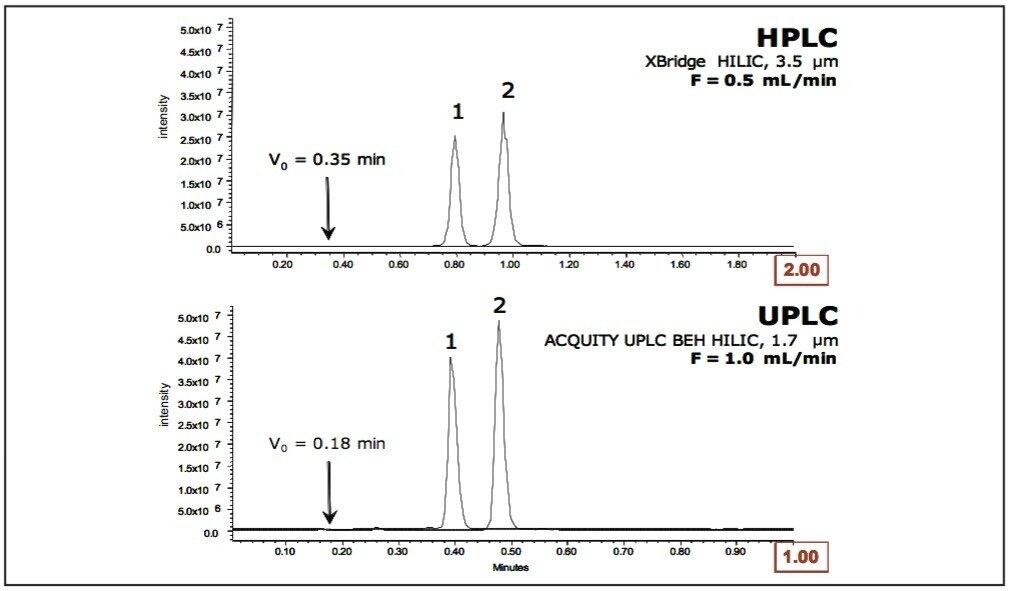
Another advantage to using BEH particles for HILIC is that the separations are directly scalable between HPLC and UPLC technology (Fig. 6). ACQUITY UPLC BEH HILIC Columns are built using the same BEH particle technology as XBridge HILIC HPLC Columns, which allows easy transfer of HILIC methods between both technology platforms.
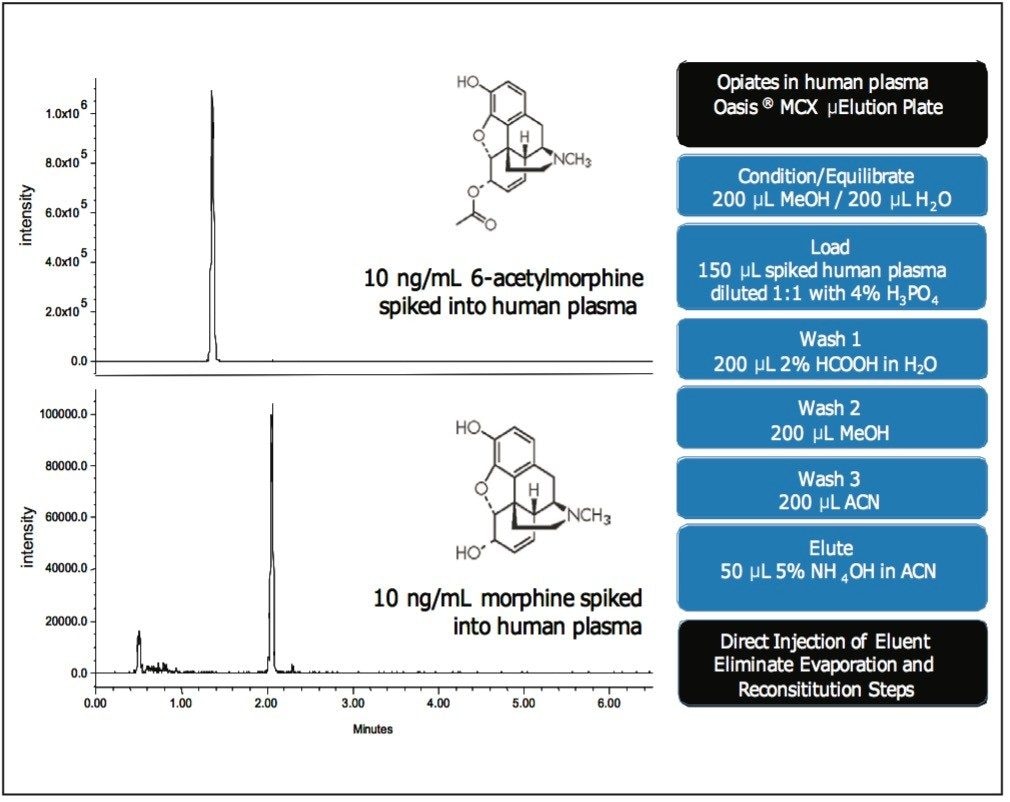
Methods that utilize SPE for sample preparation traditionally contain an elution step with high organic solvent. In order to make this eluate compatible with RP-HPLC, the extract must be evaporated and reconstituted in a more compatible solution (usually the starting mobile phase), or diluted with water. In HILIC, the highly organic eluent from SPE can be directly injected onto the column (Fig. 7), thus eliminating the need for dilution or evaporation, which is usually a rate-limiting step in increasing sample throughput.
Novel BEH particles were found to be useful for HILIC separations. They exhibited chemical resistance superior to that of silica particles at moderate pH over the course of 2,000 injections. Adding small amounts of alternative polar solvents enhanced retention of highly polar compounds. HILIC offers complementary selectivity and was found to yield up to 10-fold improvement in ESI-MS response when compared to RP-HPLC. Separations were found to be directly transferable between XBridge HILIC and ACQUITY UPLC BEH HILIC Columns. Finally, direct analysis of SPE eluates is possible by HILIC without the need for dilution or time-consuming evaporation and reconstitution.
720002711, July 2008