
In this application note, we describe the use of innovative software, Waters IntelliStart, and hardware tools, the ACQUITY TQD tandem mass spectrometer, to rapidly develop an MRM method for the non-steroidal anti-inflammatory drug (NSAID) ibuprofen with subsequent incorporation into an LC-MS/MS method for separation, detection, and quantitation.
The combination of ESCi Technology and IntelliStart Software provides an efficient, rapid, and effective approach to developing MRM methods for bioanalytical assays
Liquid Chromatography coupled with tandem quadruple mass spectrometry (LC-MS/MS) and operated in multiple reaction monitoring (MRM) mode is often the analytical method of choice for the determination and quantification of drugs and their metabolites in biofluids and tissues. This is due to the strong specificity, and therefore high levels of sensitivity, that LC-MS/MS is capable of achieving.
However, the use of this technique often requires companies to hire LC-MS specialists, or to train current personnel, both of which represent significant investments in time and resources. Developing a robust MRM method can be time consuming and requires a high level of expertise in order to make informed decisions.
The development of user-friendly and intuitive software tools that can assist or completely automate the operation of LC-MS/MS instrumentation can provide an essential service in several ways:
In the development of an LC-MS/MS method, the analyst must first optimize the operational parameters for the mass spectrometer. Traditionally this has required a high level of expertise in mass spectrometry instrumentation. Parameters that must be determined for successful mass spectrometric detection and quantitation of a chosen compound are:
Determining the optimum operating conditions generally requires some trial and error, which, for non-experts, can be time-consuming and daunting. In particular, choosing the optimum ionization mode has involved physically changing the source.
In this application note, we describe the use of innovative software, Waters IntelliStart, and hardware tools, the ACQUITY TQD tandem mass spectrometer, to rapidly develop an MRM method for the non-steroidal anti-inflammatory drug (NSAID) ibuprofen with subsequent incorporation into an LC-MS/MS method for separation, detection, and quantitation.

|
LC system: |
ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC BEH C18 Column, 2.1 x 50 mm, 1.7 μm |
|
Column temp.: |
40 °C |
|
Flow rate: |
450 μL/min |
|
Mobile phase A: |
0.1 % Ammonium Hydroxide in H2O |
|
Mobile phase B: |
MeOH |
|
MS system: |
Waters TQ Mass Spectrometer |
|
Ionization mode: |
ESI negative |
|
Capillary voltage: |
3.8 KV |
|
Cone volyage: |
15 V |
|
Desolvation temp.: |
450 °C |
|
Desolvation gas: |
900 L/Hr |
|
Source temp: |
150 °C |
|
Acquisition mode: |
MRM Transition 205 > 161 |
|
Collision energy: |
10 eV |
A generalized workflow for the development of an MRM method in bioanalysis is shown in Figure 2. These are the steps that are generally taken to determine the correct parameters for running an MS method subsequent to LC separation.
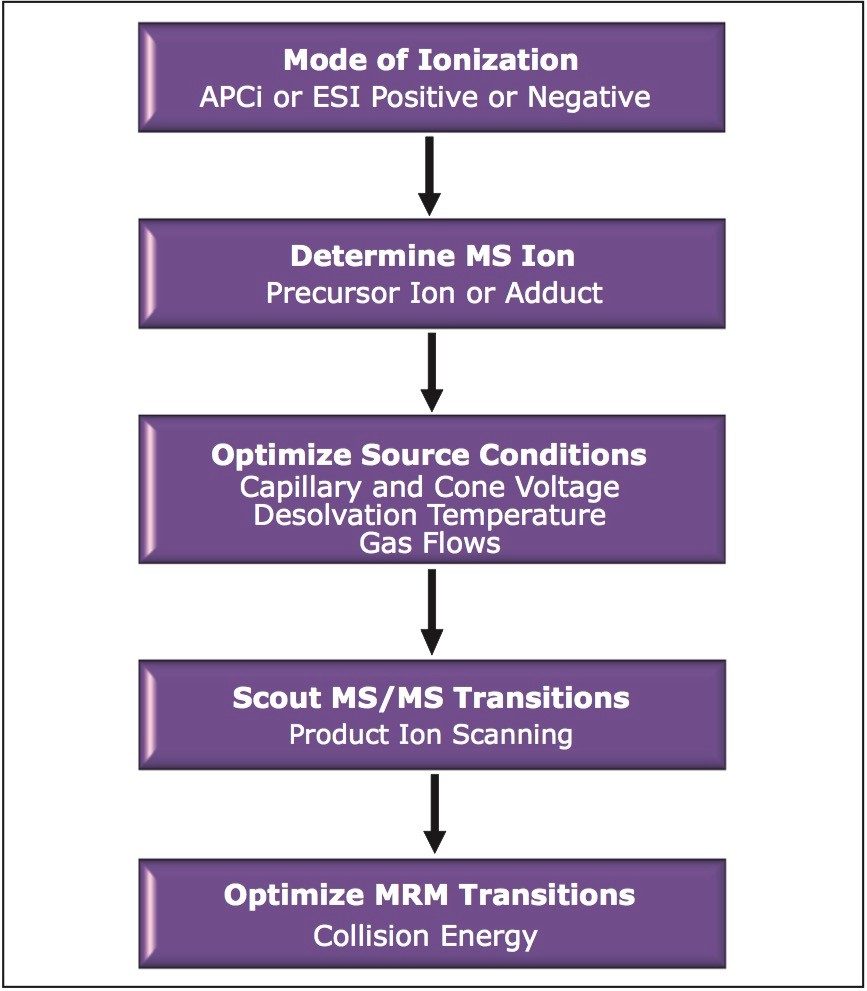
This process is both simplified and automated by using ESCi Technology and IntelliStart Software. ESCi is an ionization techniqu that allows both positive and negative ion APCi and ESI to be carried out simultaneously – without physical changes to the ion source. When used in conjunction with IntelliStart, a software tool designed to facilitate fast and accurate optimization of parameters for MRM method development, ESCi greatly reduces the amount of time involved in optimizing the operating parameters for the mass spectrometer. Figure 3 illustrates the few steps required to quickly develop an MRM method.
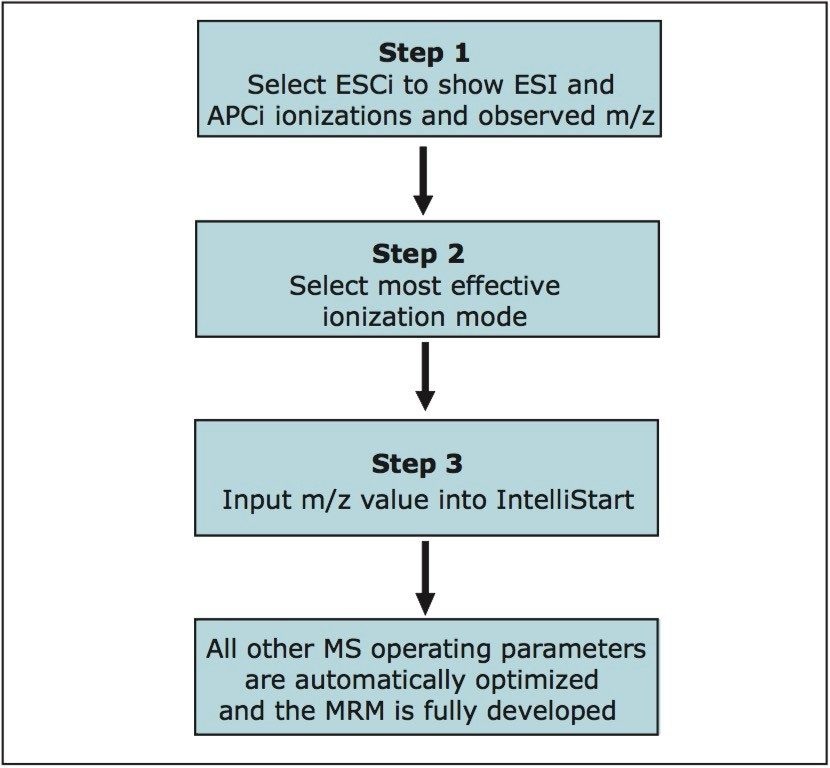
Using ESCi in Steps 1 and 2 of the optimization process allowed the user to determine the best ionization source for analysis of the compound in less than one minute and three keystrokes, as shown in Figure 4.
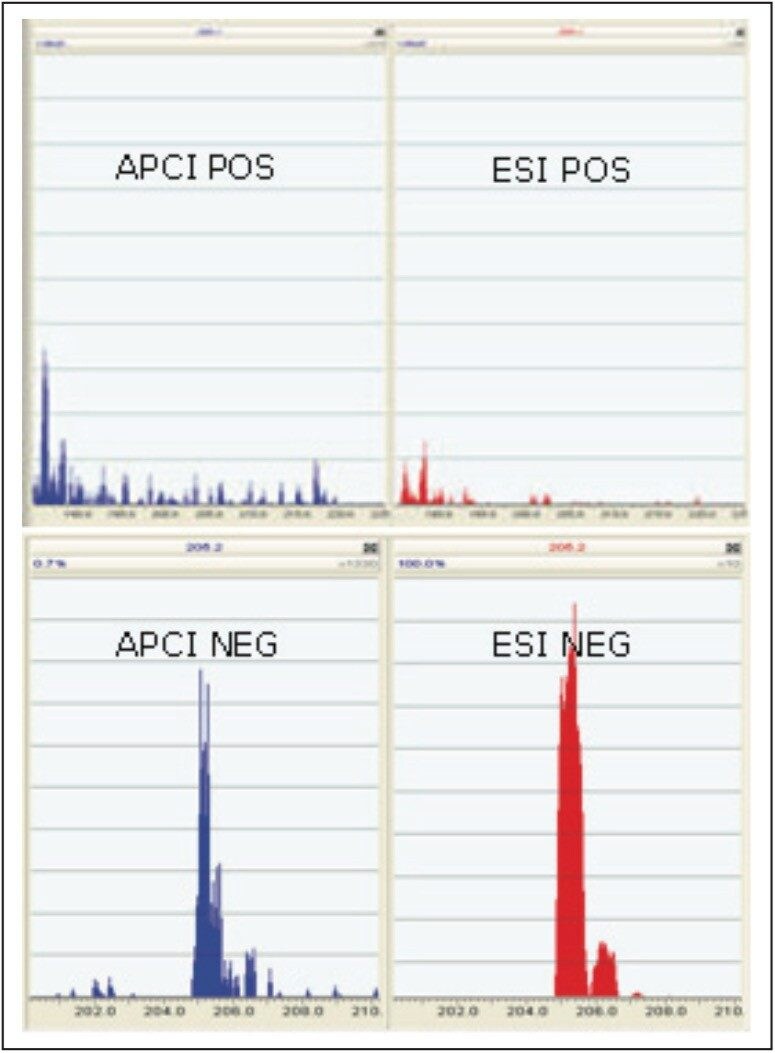
Figure 4 clearly illustrates that ibuprofen ionized in both APCi and ESI negative modes; ESI negative yielded the best sensitivity and was therefore chosen. This step in the optimization process also told us the molecular ion observed was m/z 205.
With the use of IntelliStart Software, the critical parameters for optimization of the MS method could now be obtained.
The molecular ion mass was entered into IntelliStart (Step 3) and the default ranges for cone voltage and collision energy were utilized to enable IntelliStart to automatically determine all other parameters, such as optimized voltages, desolvation temperatures, gas flows, and MRM transition, all in less than 5 minutes.
A report was automatically generated specifying the optimized settings for the MRM method. The report also detailed the daughter ion spectra along with cone voltage and collision energy values, as shown in Figure 5.
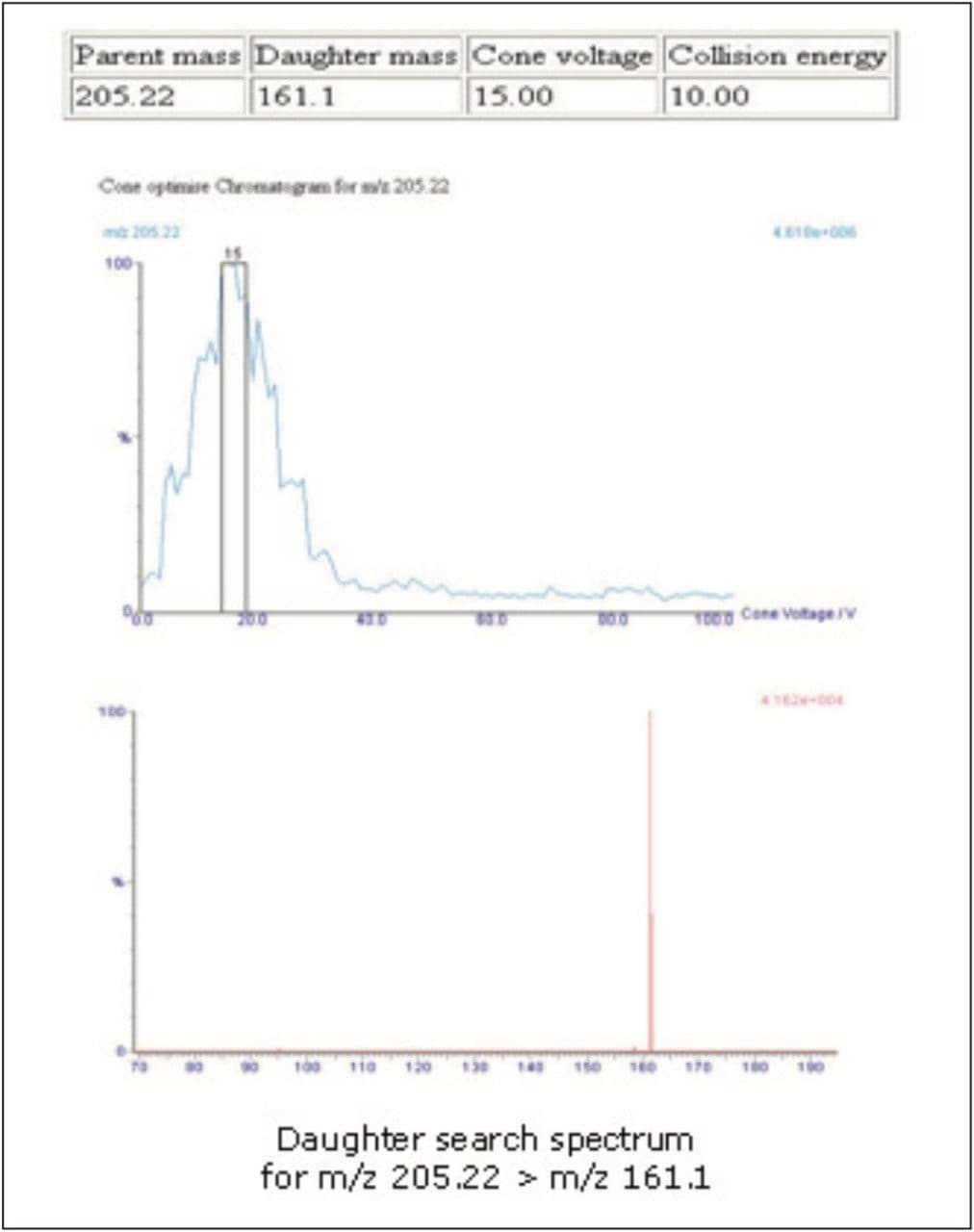
Having successfully and quickly developed the MS method for the detection of ibuprofen, the MRM method parameters determined by IntelliStart are now inserted into the instrument method (Figure 6) for subsequent use in the method for optimizing the chromatographic conditions for ibuprofen. Because data are automatically transferred, transcription errors are avoided and time is saved. IntelliStart is available for both Empower and MassLynx software platforms.
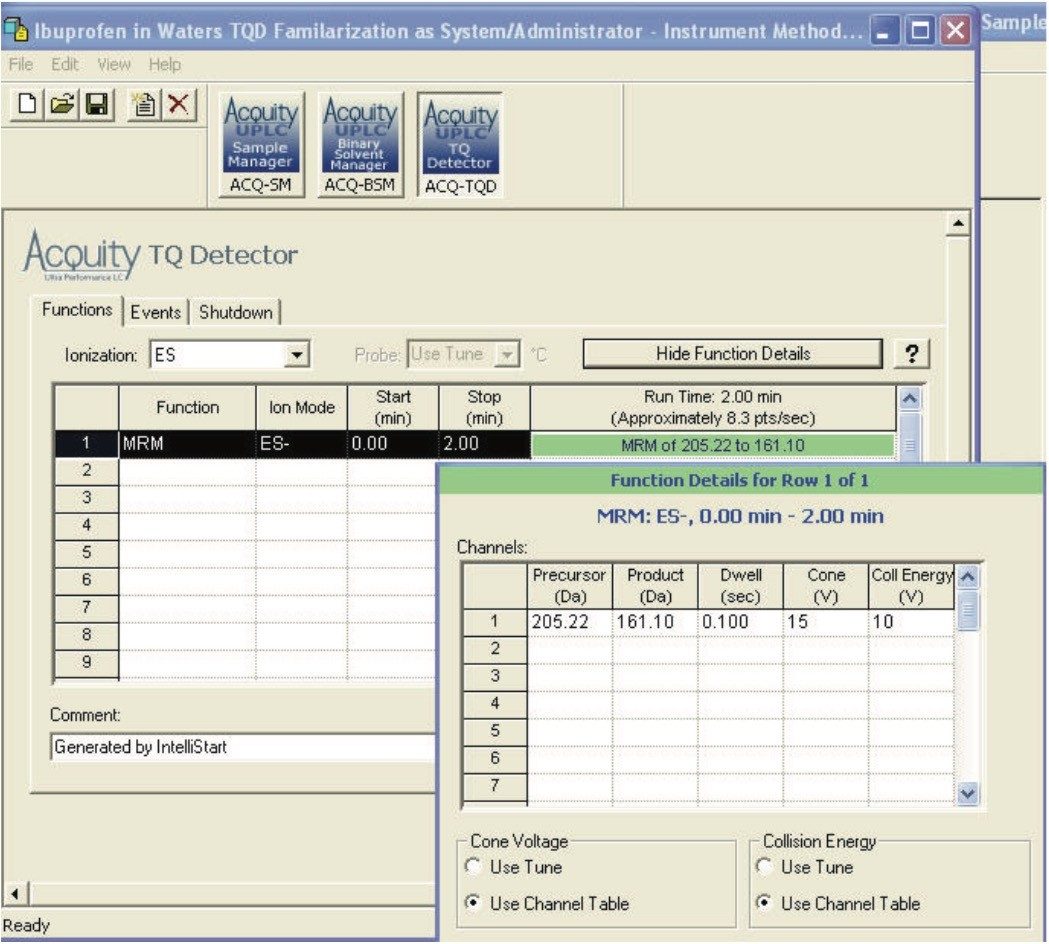
A complete LC-MS/MS method for the determination of ibuprofen in biological fluids can now be developed, with all the optimized parameters for detection and quantitation in place, as shown in Figure 7.
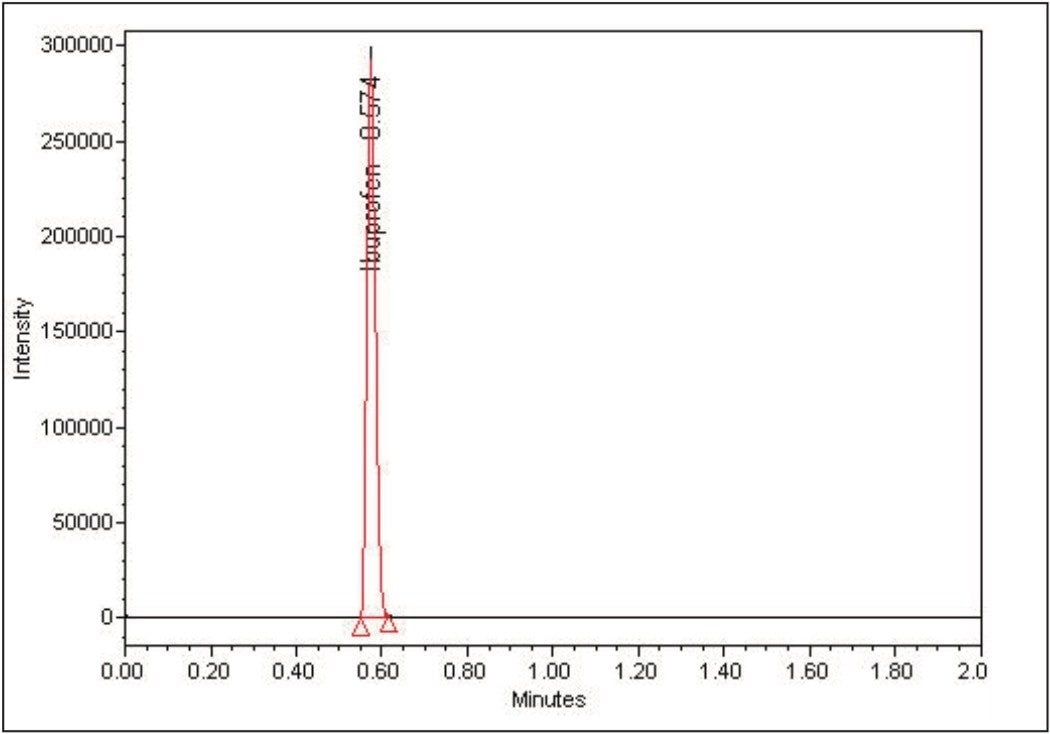
The combination of ESCi Technology and IntelliStart Software provides an efficient, rapid, and effective approach to developing MRM methods for bioanalytical assays.
720002569, March 2008