In this application note, we have demonstrated that the Xevo TQ MS can acquire full-scan and MRM channel data to determine the level of a model pharmaceutical compound in urine and its metabolite information in a single analysis.
The measurement of the levels of circulating drugs and their metabolites is important information in the development of new therapies. Drug levels in biofluids are used to determine the bioavailability of a drug. Additionally, elucidation of drug metabolite information is vital due to the fact that they can often be toxic at certain levels, have a greater pharmacodynamic effect than the parent drug, interfere with concomitant medication, and impact liver function.
These two different pieces of information are normally acquired in separate analytical experiments, resulting in increased laboratory workload and reduced efficiency. Therefore the ability to determine drug concentration and obtain metabolite structural information during a single analysis is not only faster but more cost effective. In the case of low sample volumes, e.g., pediatric studies, this capability is critical for laboratories to obtain required quantitative and qualitative data.
The Waters Xevo TQ Mass Spectrometer is a tandem quadrupole system equipped with a novel collision cell design that allows fullscan MS and quantitative multiple reaction monitoring (MRM) data to be acquired in a single analytical run.
Here, we present a method whereby full-scan MS and MRM data can be acquired in a single run to determine the levels of a model pharmaceutical in urine and utilize the associated full-scan data to determine its related metabolites.

Human urine was collected from volunteer individuals eight hours after dosing with 400 mg of ibuprofen. The samples were stored frozen prior to analysis. Samples were prepared by centrifugation at 13,000 RCF for 5 minutes and diluted with water. Samples were then injected onto the UPLC-MS/MS system.
|
LC system: |
Waters ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC BEH C18 Column, 2.1 x 50 mm, 1.7 μm |
|
Column temp.: |
40 °C |
|
Flow rate: |
600 μL/min |
|
Mobile phase A: |
0.1% NH4OH |
|
Mobile phase B: |
ACN |
|
Gradient: |
5% to 95% B/2 min |
|
MS system: |
Waters Xevo TQ MS |
|
Ionization mode: |
ESI negative |
|
Capillary voltage: |
2000 V |
|
Cone voltage: |
15 V |
|
Desolvation temp.: |
550 °C |
|
Desolvation gas: |
1000 L/Hr |
|
Source temp.: |
150 °C |
|
Scan range: |
m/z 100 to 500 |
|
Collision energies: |
MRM data 7 V, full-scan data 3 V |
|
MRM transition: |
m/z 205 > 161 |
Determining drug concentration and drug metabolites are both important aspects in developing a new medicine. This experiment was designed such that the levels of ibuprofen in urine were measured by MRM mass spectrometry and full-scan MS data was collected to detect the associated metabolites during a single injection.
The unique collision cell design of the Xevo TQ MS, which is continuously filled with collision gas, enables it to operate with rapid switching between MS and MS/MS data acquisition modes. This occurs in timeframe that is compatible with the fast chromatography and narrow peaks generated by the ACQUITY UPLC System: the Xevo TQ MS is capable of operating at up to 10,000 Da/sec and can correctly define the very sharp peaks produced by UPLC.
In this dataset, greater than 12 scans were acquired for the MRM channel of ibuprofen while also obtaining full scan MS data. Peaks widths were on the order of 2.4 seconds measured at peak base (data not shown).
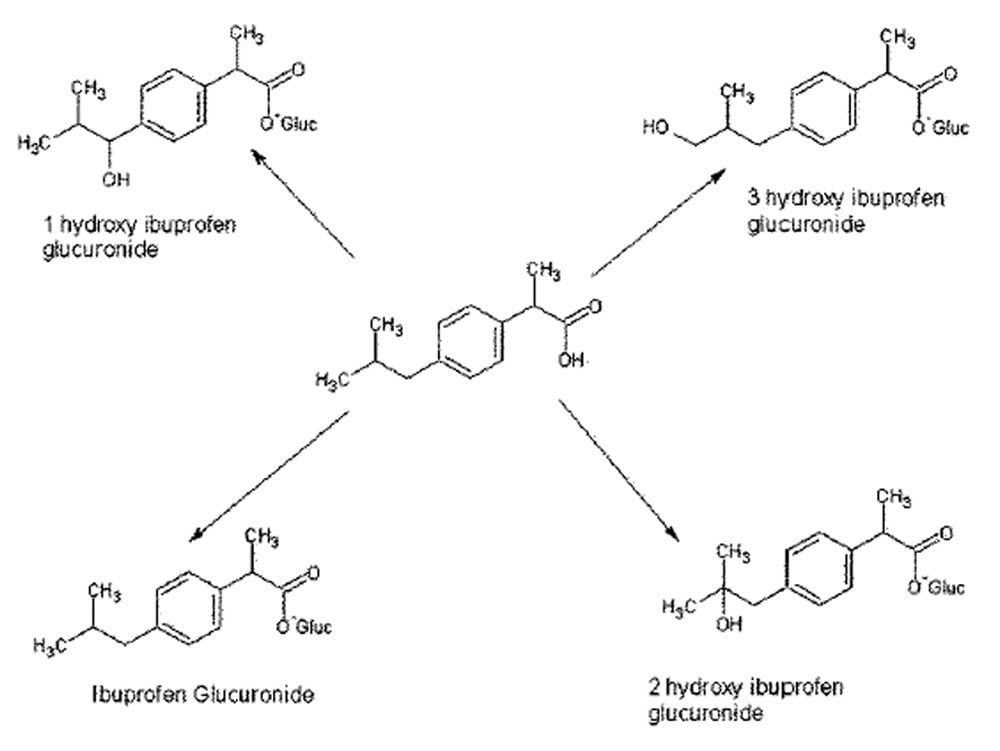
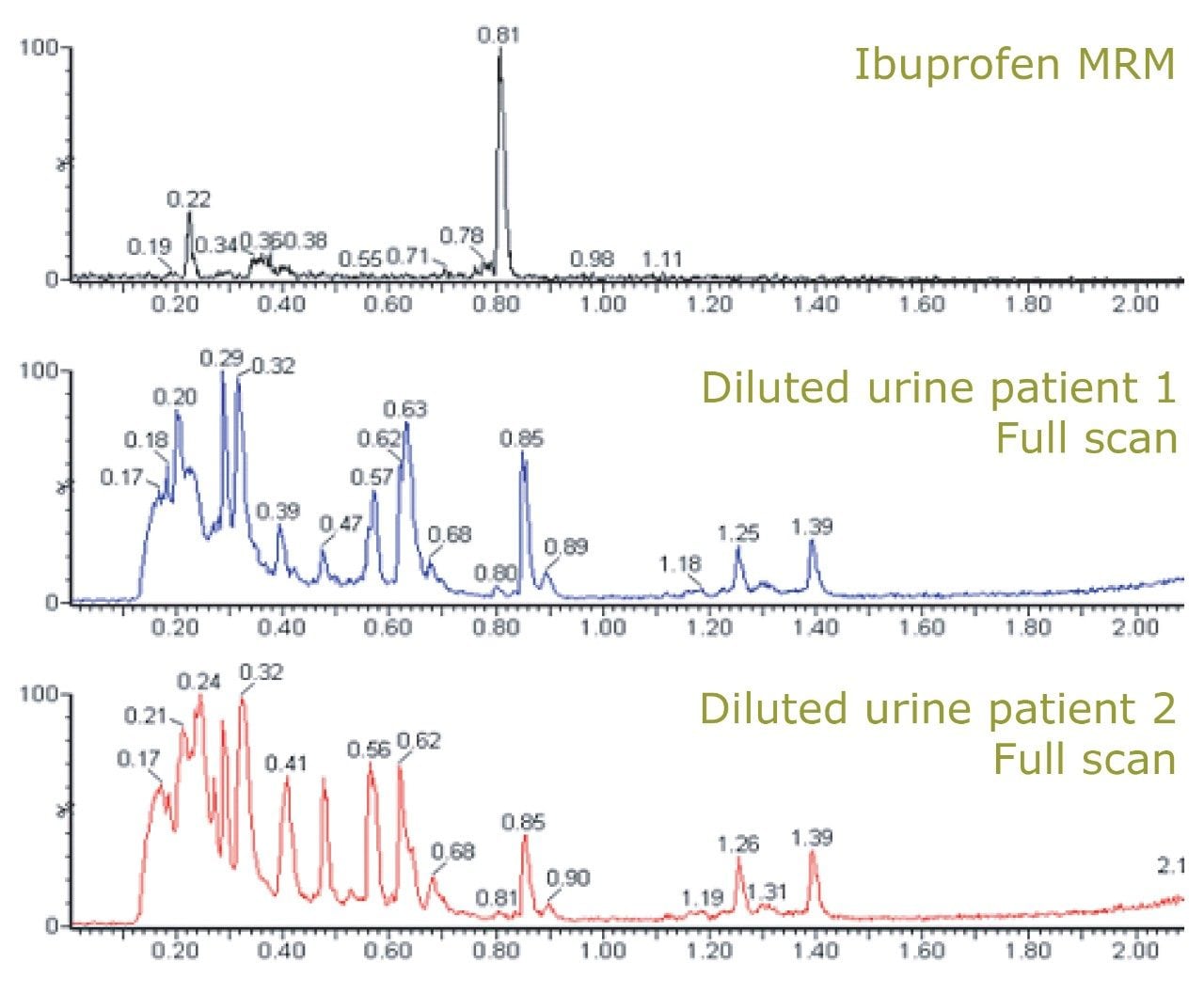
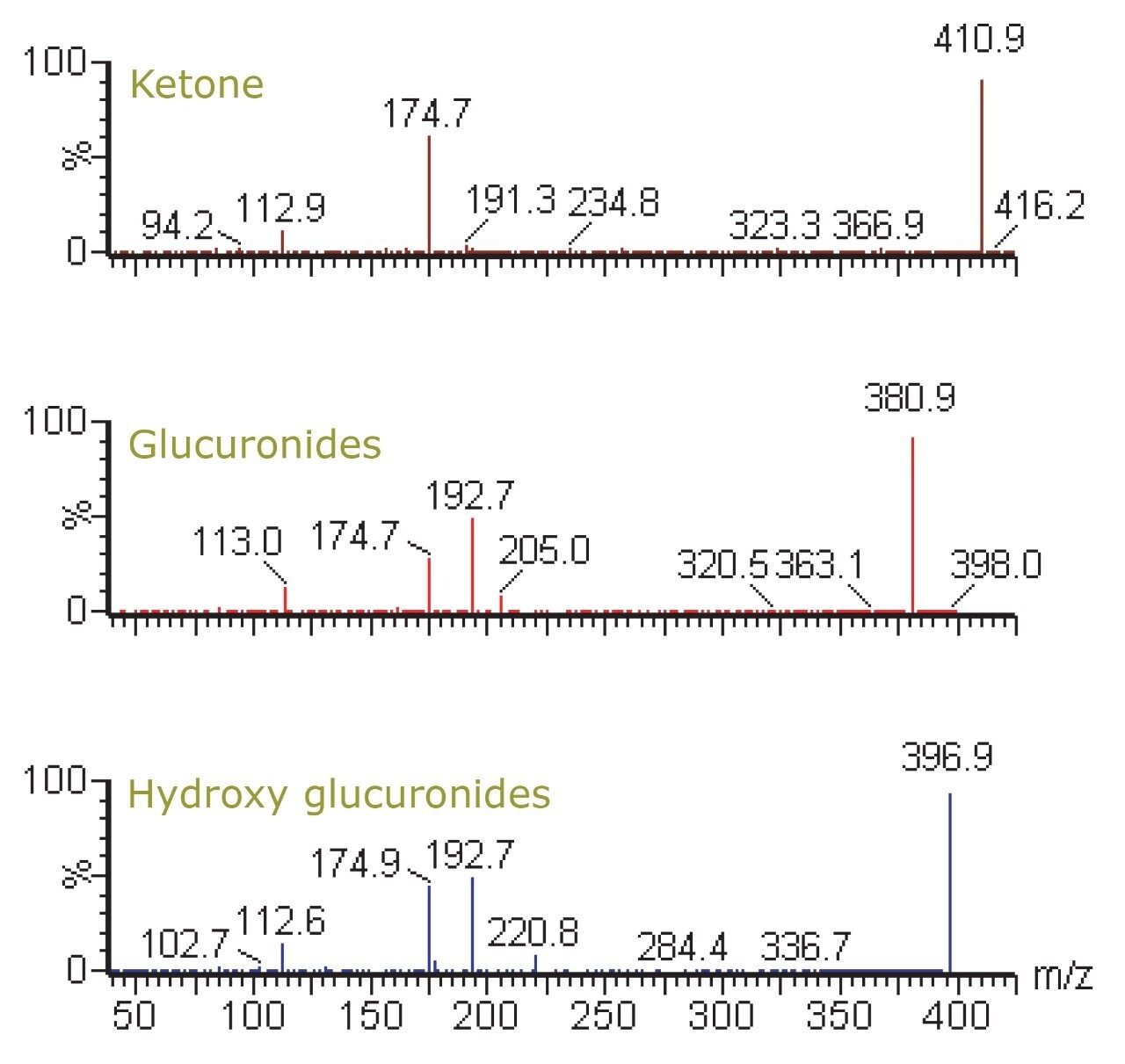
Figure 2 shows the chemical structure of ibuprofen and some of its major in vivo metabolites. Figure 3 displays the MRM transition data for ibuprofen in the urine samples and also the simultaneously acquired full-scan data. The full-scan data were then mined for potential metabolites resulting from ibuprofen. Figure 4 shows extracted ion chromatograms (XIC) that were generated relating to the ketone glucuronide (m/z 411), glucuronide (m/z 381), and hydroxy glucuronide (m/z 397) metabolites.
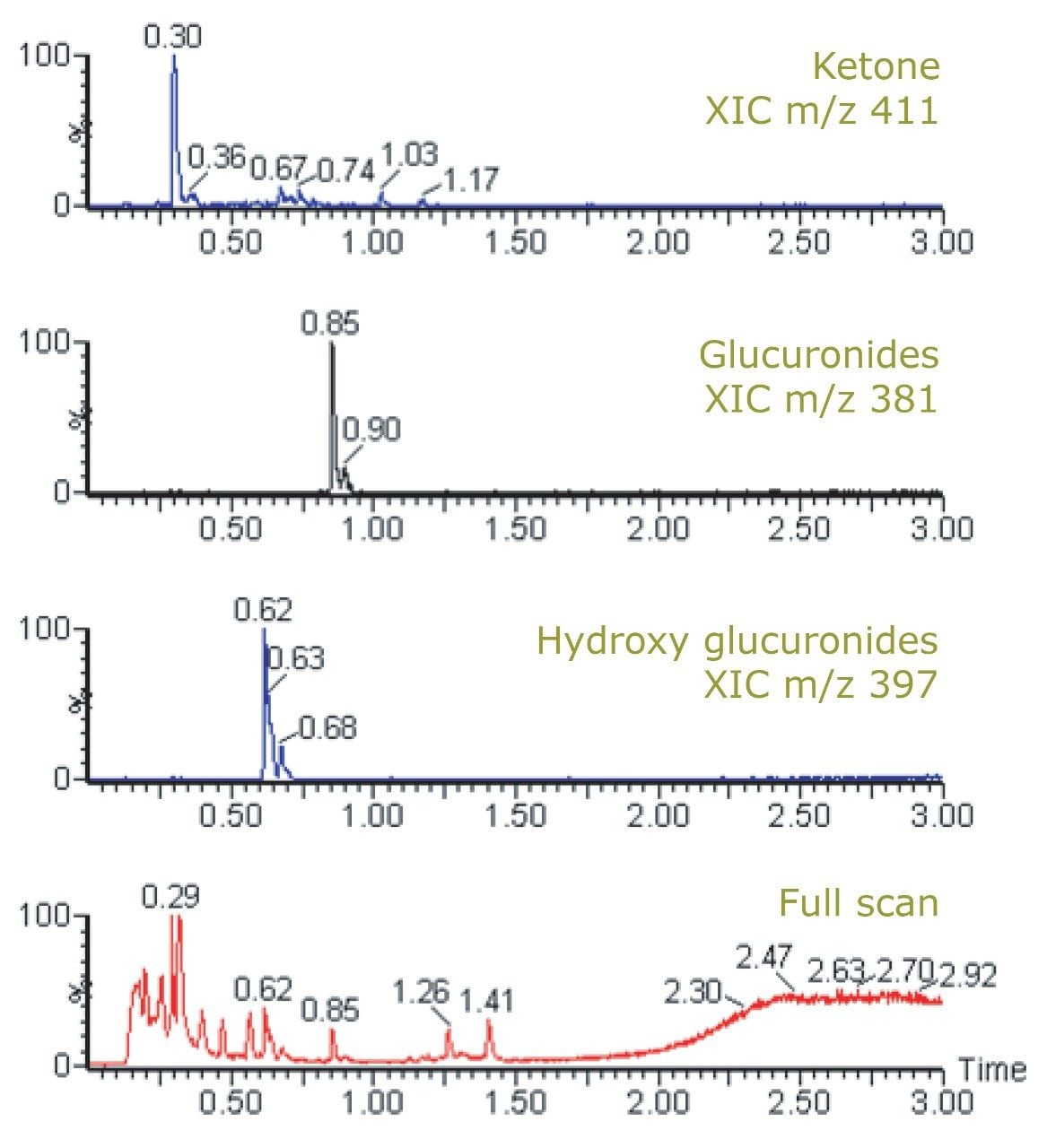
Further confirmatory product ion MS experiments revealed several diagnostic fragment ions, such as m/z 193 and 175 for the glucuronide acid moieties, m/z 221 for the aglycone, and m/z 113 for ibuprofen itself (Figure 5).1
A further advantage to this acquisition approach is that it provides the scientist with the ability to visualize the differences between subject matrix using the full-scan MS. These differences may be related to several factors: diet, sex, age, or the state of an individual’s health. Thus the full scan data could be additionally utilized for the detection of biomarkers. Further, the full-scan MS data could be interrogated in the future if new information is required about the metabolism of the compound – without the need to re-run the samples.
In this application note, we have demonstrated that the Xevo TQ MS can acquire full-scan and MRM channel data to determine the level of a model pharmaceutical compound in urine and its metabolite information in a single analysis. The speed of the Xevo TQ MS proves to be highly compatible with the high resolving power of the ACQUITY UPLC System.
The benefits of this technique are realized in several ways. First, the ability to gather full-scan data along with MRM channel data enables scientists to collect multiple dimensions of information about a sample in a single run – maximizing the resource utilization of a laboratory that otherwise would have been performing multiple experiments to gain the same information. Second, coupling this MS technique with UPLC ensures a faster analysis. Finally, the richness of the data acquired by full-scan MS allows that information to be mined in multiple ways, giving researchers more confidence in their decisions as they direct their drug discovery and development studies.
720002832, October 2008