This application note demonstrates the feasibility of analyzing bromate ions in tap water using the HILIC separation mode and flexible LC-MS instrumentation that already exists in many laboratories worldwide.
Bromates found in drinking water pose a toxic health risk to humans if ingested in significant quantities. Even at very low concentration, the presence of bromate in drinking water has the potential for adverse long-term health effects. Bromate is not endogenous to natural sources of water; however, bromate contamination is created as a by-product of advanced ozone water treatments. Ozone reacts with natural sources of elemental bromine found in water supplies and the level of bromine can vary from source to source. To date, there are no practical methods for removing the bromine or the bromate by-product, and the only solution is to limit the bromate formation during the water treatment process. This requires careful monitoring of the bromate concentration so that it does not exceed safe drinking water standards. The World Health Organization (WHO) drinking quality standards recommendation is limited to 10 ppb (μg/L) bromate in drinking water.1
All standard solutions were prepared using 90% acetonitrile:10% de-ionized water. Samples of tap water were prepared by diluting 5-fold with acetonitrile.
|
LC System: |
ACQUITY UPLC |
|
Detector: |
ACQUITY SQD (-ve ESI, SIR mode m/z 128.9) |
|
Column: |
ACQUITY UPLC BEH Amide, 2.1 x 100 mm, 1.7 μm, part number 186004801 |
|
Column Temp.: |
40 °C |
|
Injection Volume: |
20 μL |
|
Injection Mode: |
Full loop |
|
Flow Rate: |
0.3 mL/min |
|
Isocratic Mobile Phase: |
90% acetonitrile: 10% 50 mM aqueous ammonium formate (unbuffered) |
|
Weak Needle Wash: |
Acetonitrile |
|
Strong Needle Wash: |
Water |
|
Seal Wash: |
50% acetonitrile:50% water |
|
Data Management: |
MassLynx, version 4.1 |
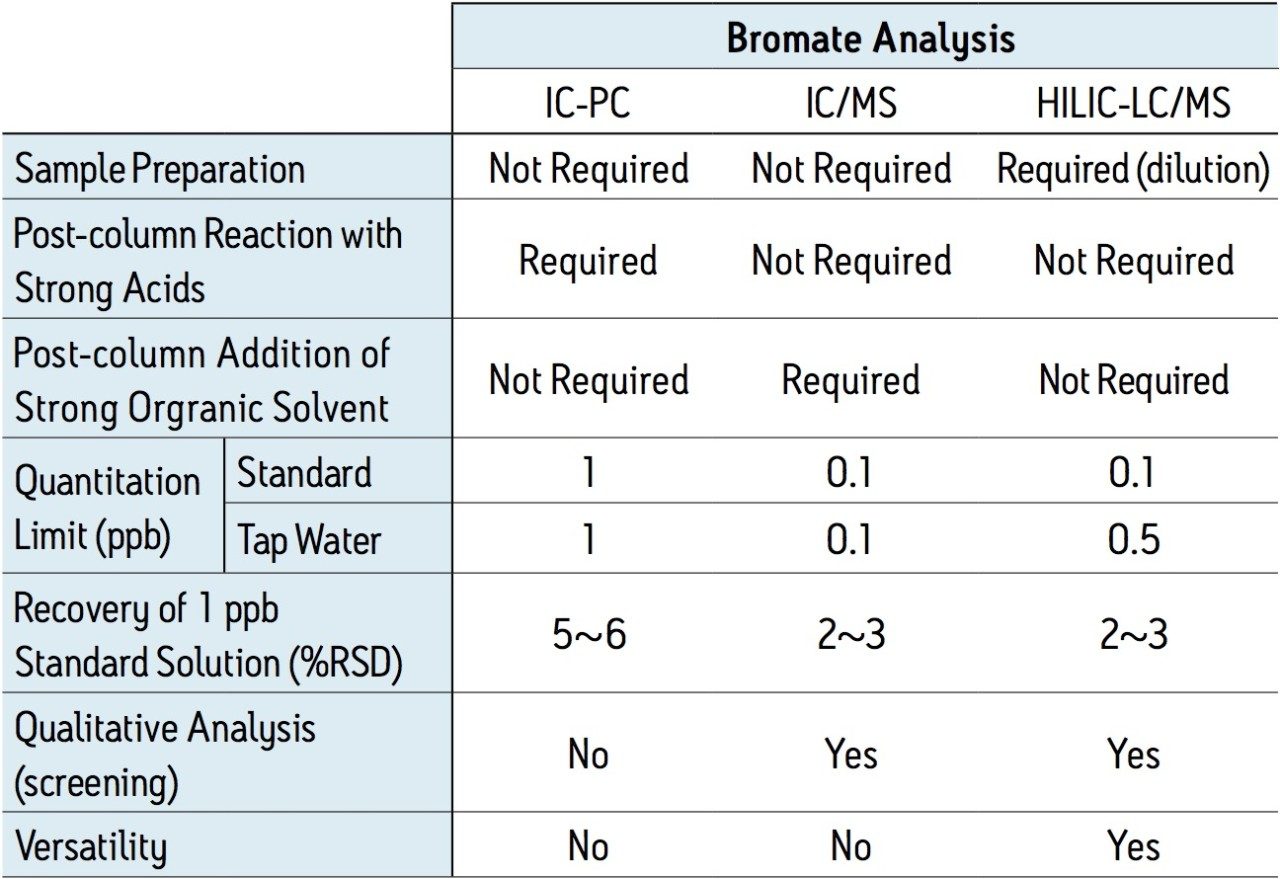
To establish reliable data, it is recommended that the analytical method be at least 10-fold more sensitive than the action limit imposed for the assay. By following the 10 ppb WHO guideline for bromate, this would require that the analyte be detectable at 1 ppb or lower. Typical IC methods can detect bromate at these very low levels; however, the methods used for IC are more labor intensive and require a rigorous instrument maintenance schedule due to the reagents used for the post-column reaction. In contrast, HILIC chromatography coupled with MS detection provides both ease of use and high sensitivity. Figure 1 shows single ion recording (SIR) MS chromatograms in the range of 0.05 – 1 ppb. The mass-to charge ratio monitored for the bromate ion was 128.9 Da.
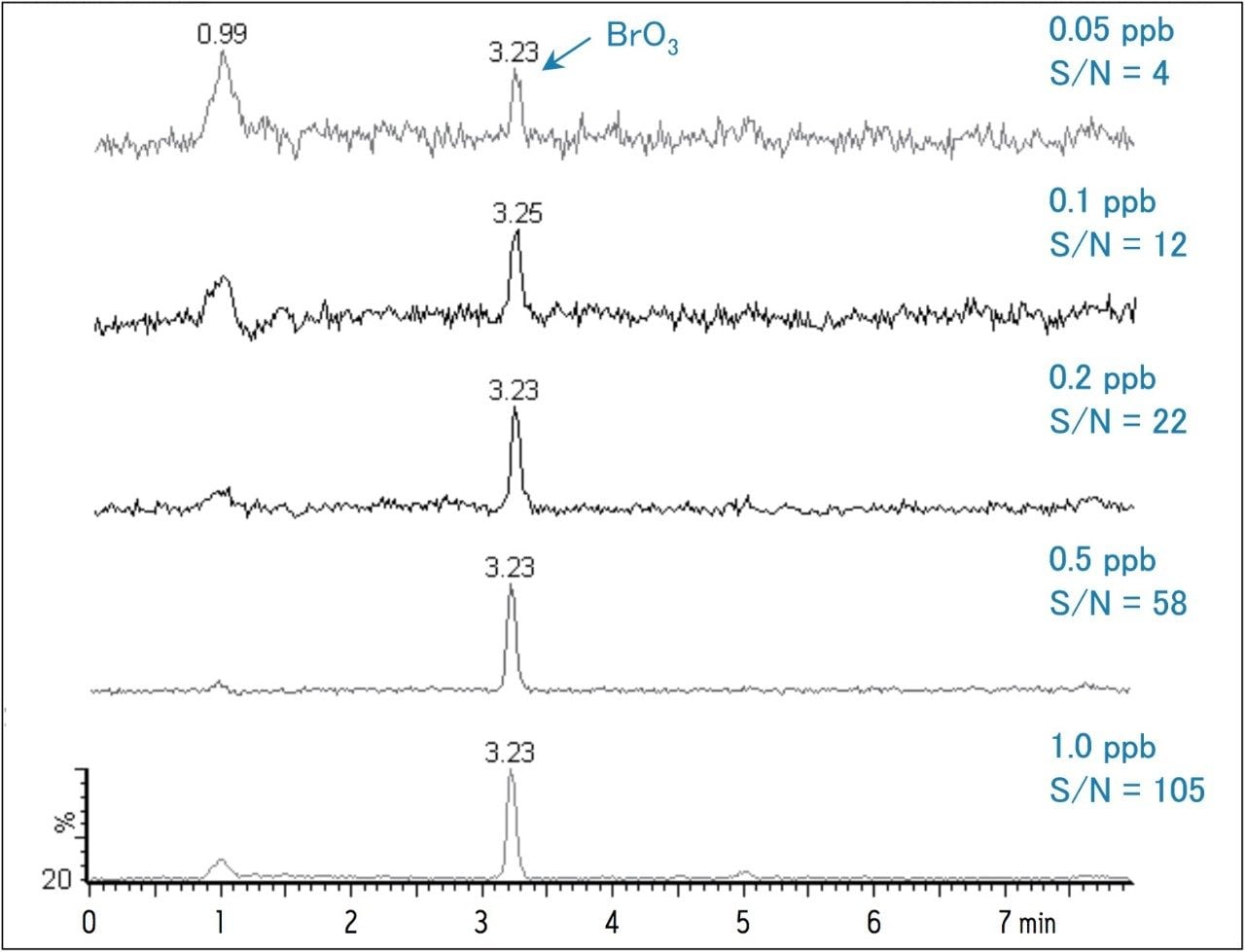
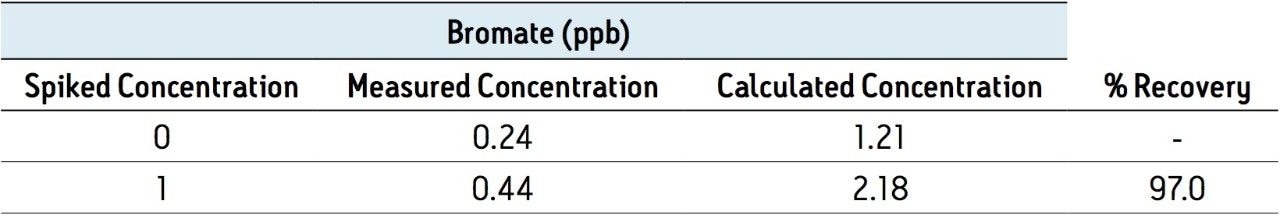
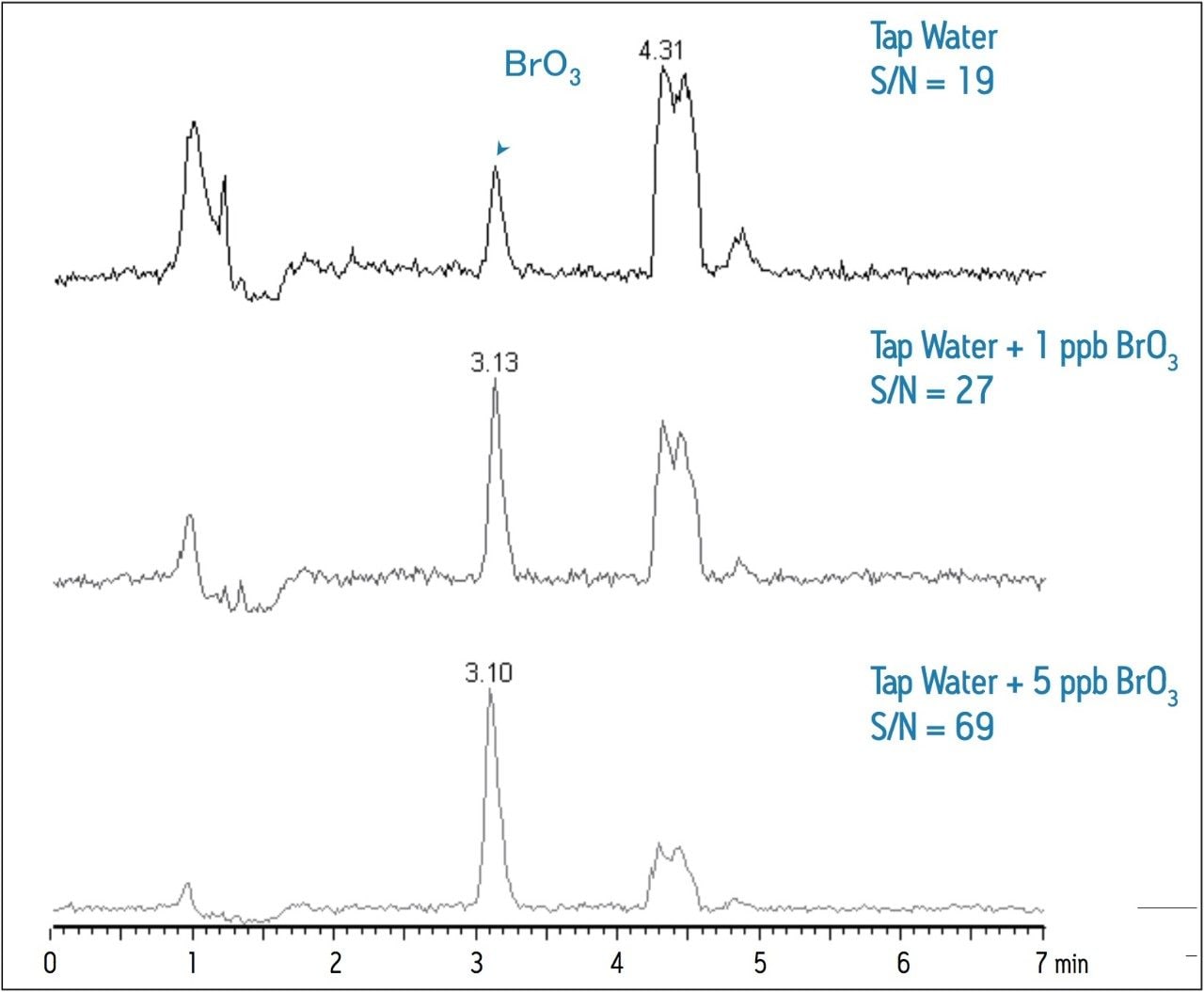
Table 2 summarizes the recovery data of a spiking experiment in tap water. Figure 2 provides detailed chromatograms for the spiking experiment. The recovery of bromate in the tap water was greater than 95% for both spike levels.
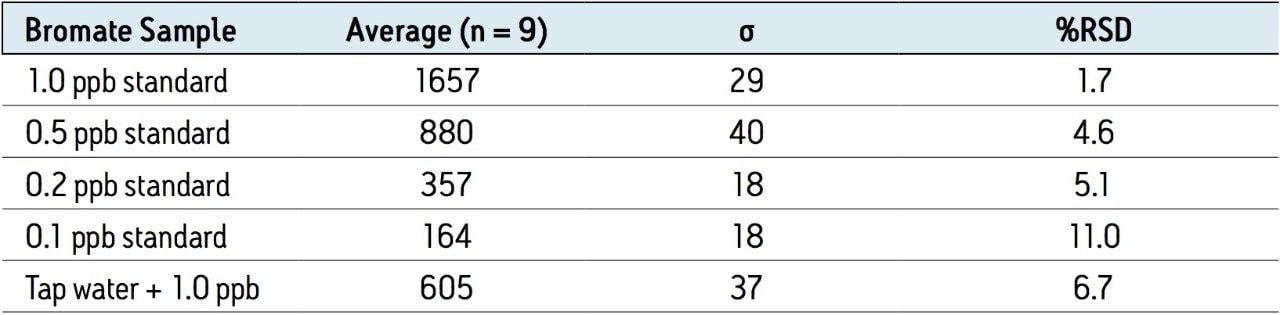
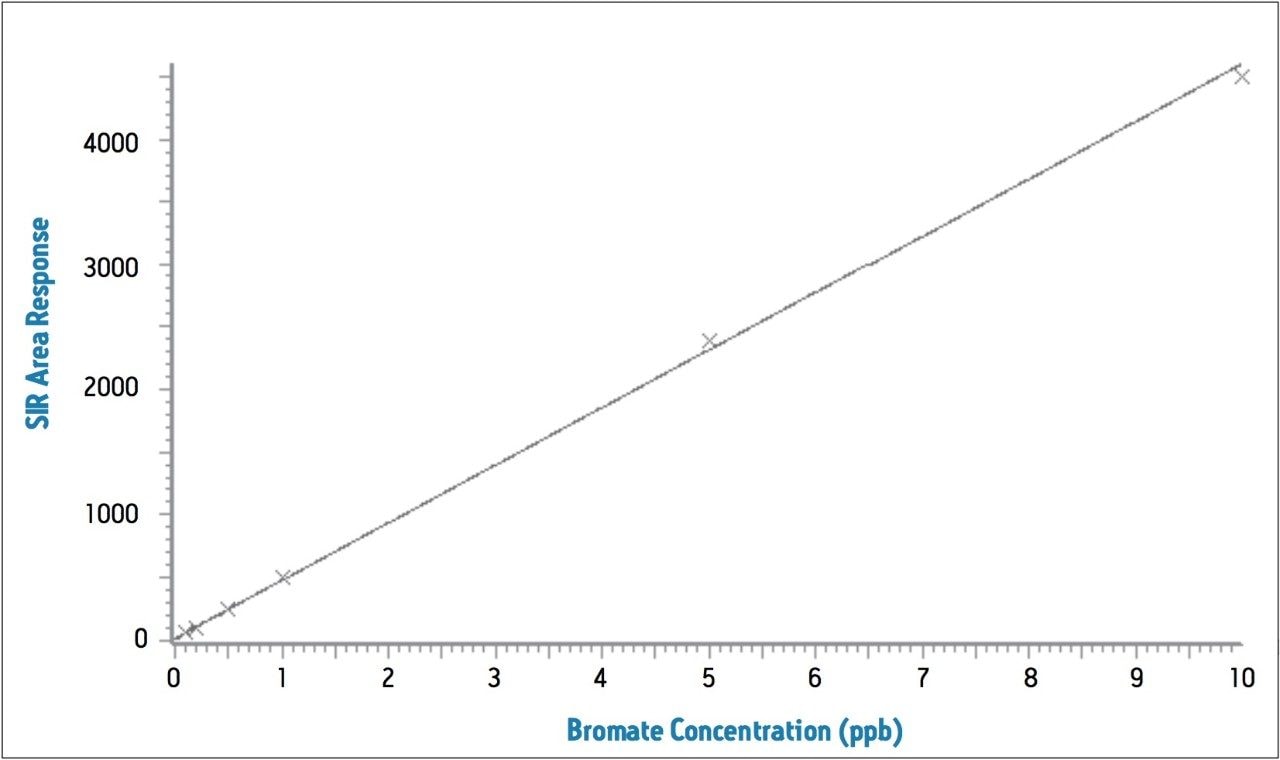
The reproducibility data for standard bromate solutions and tap water spiked with 1 ppb of bromate (N=9) is shown in Table 3. The relative standard deviation of the 1 ppb standard bromate solution and a 1 ppb spiked water sample is 1.7% and 6.1%, respectively, and shows good linearity over the spiking range (Figure 3).
The sensitivity offered by the HILIC-LC/MS method was found to be highly effective for the trace level detection of bromate ions in tap water at levels near 0.1 ppb without the need for post-column derivatization of the sample. This application demonstrates the feasibility of analyzing bromate ions in tap water using the HILIC separation mode and flexible LC-MS instrumentation that already exists in many laboratories worldwide.
720004197, February 2012