
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates to improve the method robustness for the analysis of amoxicillin tablets without method revalidation.
Improvements in method robustness for UPLC analysis of pharmaceutical formulations can be achieved by choosing the correct washing steps based on the excipients present.
Formulated drug products contain not only the active pharmaceutical ingredient (API), but also binding agents, stabilizing agents and other important but pharmaceutically inactive compounds. Over time, these additional components can build up on the stationary phase and column frits causing system pressure to increase, as well as potentially negatively affect system suitability results. When an increase in system pressure is observed, it is common practice to wash the column with a high concentration of an organic solvent such as acetonitrile or methanol. However, if excipients used in a formulated drug product contain polar, aqueous soluble compounds, high percentages of organic solvent could in fact cause precipitation of these components, leading to a decrease in column performance. This technical brief focuses on incorporating a routine column washing step using an appropriate level of organic content for formulated amoxicillin tablets, and evaluates the effects of this wash step on the performance of the method. Changing the column washing procedure to effectively elute hydrophilic formulation components did not require method revalidation as the column wash is not used to analyze samples, but rather to protect method performance and robustness.
Initially, routine analysis of amoxicillin tablet formulations was performed to test the method provided by the tablet manufacturer. An ACQUITY UPLC with an ACQUITY HSS T3, 1.8 μm, 2.1 x 100 mm Column was used to test the standards and samples provided. As part of the system set-up procedure, a column wash of 50:50 acetonitrile:water was performed for 30 minutes prior to the beginning of each sample set. Sample sets consisting of 29 injections, including 15 injections of formulated sample, were run twice daily. The system was run continuously until the separation failed the requirement for theoretical plate count of the amoxicillin peak, set in the manufacturer’s standard operating procedure at no less than 5000. After approximately 350 injections, system suitability results for the amoxicillin standard failed, measuring at 4100 theoretical plates (Figure 1A). Monitoring the pressure for each run was an important part of troubleshooting the drop in plate counts. Pressure spikes for the standard injections immediately following the column washing step were observed, as well as a rapid increase in pressure over the course of the 350 injections (Figure 2). This type of pressure increase is indicative of sample build-up.
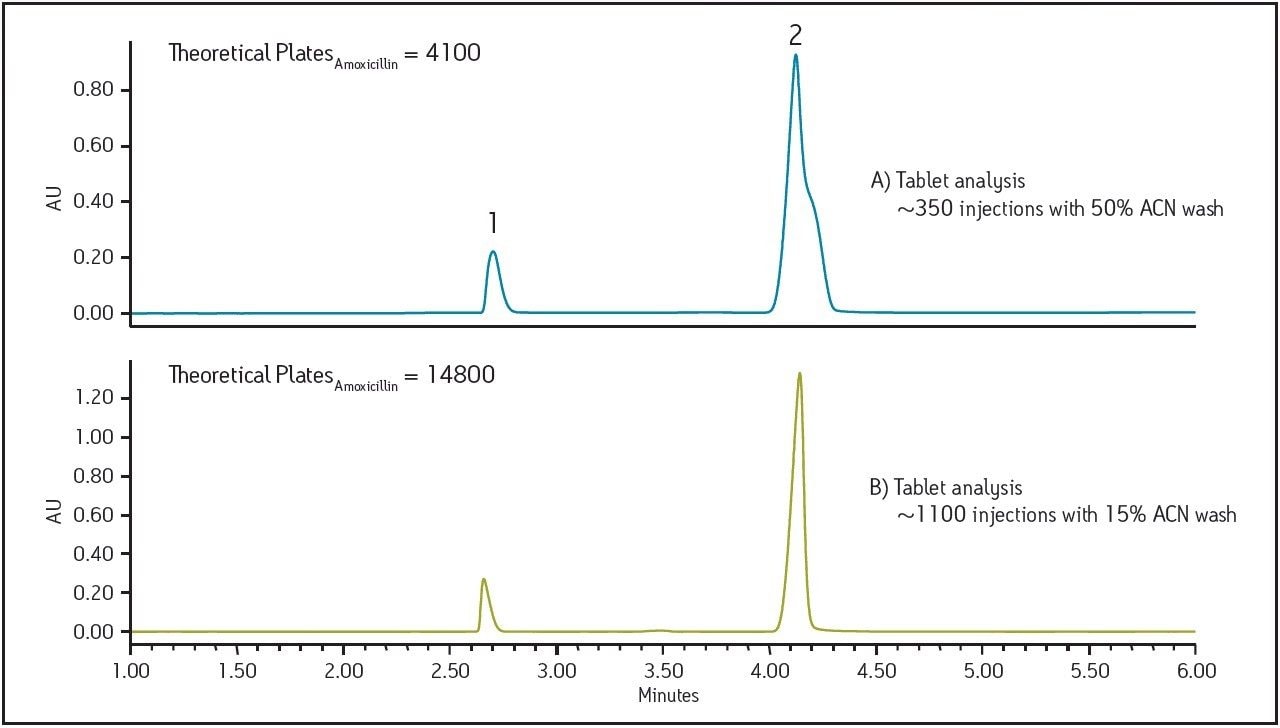
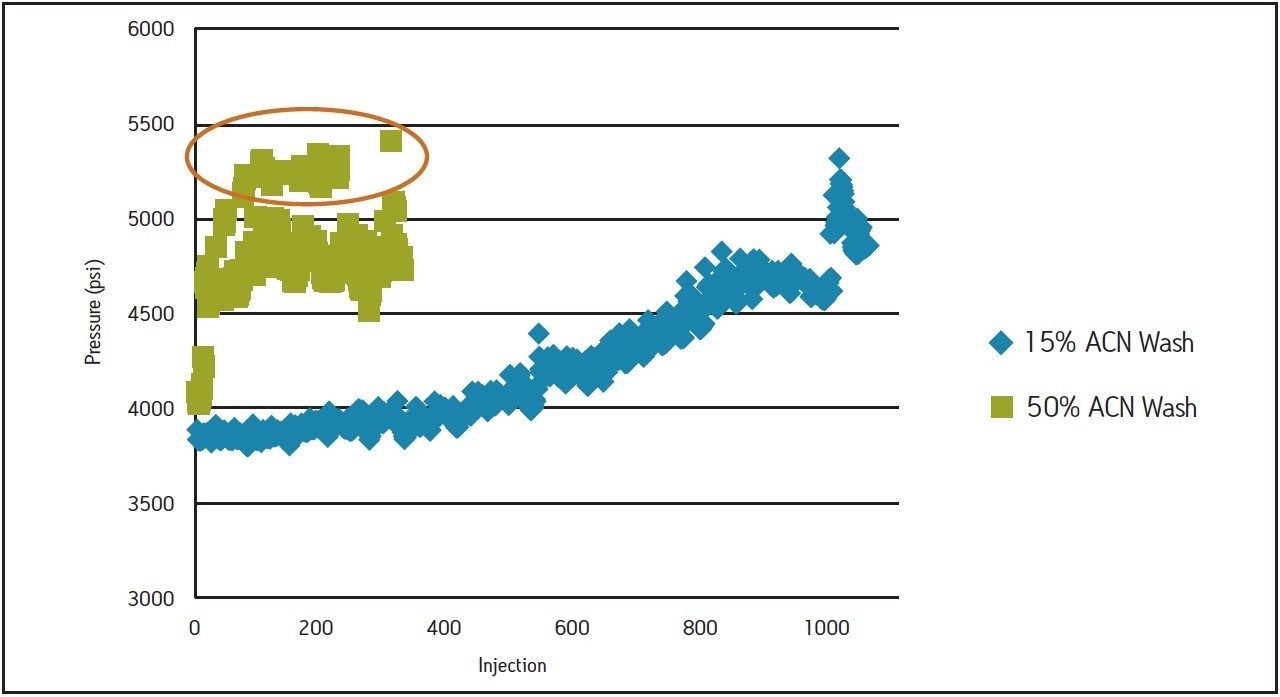
Based on the pressure increases observed postcolumn wash and an understanding of the properties of the tablet formulation, it was suspected that the wash used was incompatible with the sample. Thus the column wash was modified from 50% acetonitrile (original) to 15% acetonitrile in water. A higher aqueous wash was chosen based on the polar properties of the tablet excipients, at the same time balancing a low % organic to prevent non-polar excipients from precipitating during the wash step. Using the new wash procedure, and repeating the sample set using the manufacturer’s method on a new column, approximately 1,100 injections were performed before the experiment was halted. At 1,100 injections, the analysis was still passing the suitability criteria with 14,800 theoretical plates. (Figure 1B). An examination of the pressure data shows no pressure spikes for any runs after this modified column washing step (Figure 2). A steady, gradual increase in pressure over 1,100 injections was observed and this is to be expected with repeated injections due to the complexity and necessity of the analysis of formulated drug products. This study demonstrates that by re-evaluating and selecting an appropriate column wash composition based on the properties of the excipients in the drug product, the long term reliability of the method can be improved without the need for re-validation.
Due to the sample complexity of formulated pharmaceutical products, manufacturers may incorporate a column wash in order to minimize carryover, and protect column performance. A high percentage organic solvent column wash, which is traditionally used, may not be the best choice in all cases. If the components of the product are insoluble in organic solvents, then a high organic wash may cause the components to precipitate out of solution, increasing pressure quickly and reducing overall method performance. Understanding the composition of the sample aids in choosing the appropriate washing step, which can improve method robustness.
720004807, October 2013