RenataDX Screening System: Analytical Performance for Amino Acids, Free Carnitines and Acylcarnitines in Dried Blood Spots
For in vitro diagnostic use. Not available in all countries.
Introduction
The Waters RenataDX Screening System enables flowinjection analysis and quantification of organic compounds in biological matrices.
This document describes a test of the analytical performance of the RenataDX Screening System for the analysis of amino acids, free carnitines, and acylcarnitines in dried blood spots.

Experimental
Extracted dried blood spot (DBS) control samples were analyzed with the RenataDX Screening System, under the control of MassLynx IVD Software (v4.2), with data processed using IonLynx Application Manager.
Sample Description
A single 3-mm diameter DBS punch was incubated in a methanol-based internal standard solution. After the incubation period, the samples were transferred from the extraction plate to a clean 96-well microtitre plate.
Flow-Injection Analysis Conditions
|
System tubing: |
~1 meter PEEK (0.005" ID) with post injection valve inline filter (2 μm pore size) |
|
Mobile phase A: |
80% Acetonitrile(aq) with 0.05% (v/v) formic acid |
|
3777C wash 1: |
20% Methanol(aq) |
|
3777C wash 2: |
80% Acetonitrile(aq) with 0.05% (v/v) formic acid |
|
Flow rate: |
Variable flow rate from 150 μL/min to 15 μL/min, with 500 μL/min flush |
MS Conditions
|
Resolution: |
MS1 (0.70 FWHM), MS2 (0.70 FWHM) |
|
Acquisition mode: |
MRM |
|
Polarity: |
ESI+ |
Results and Discussion
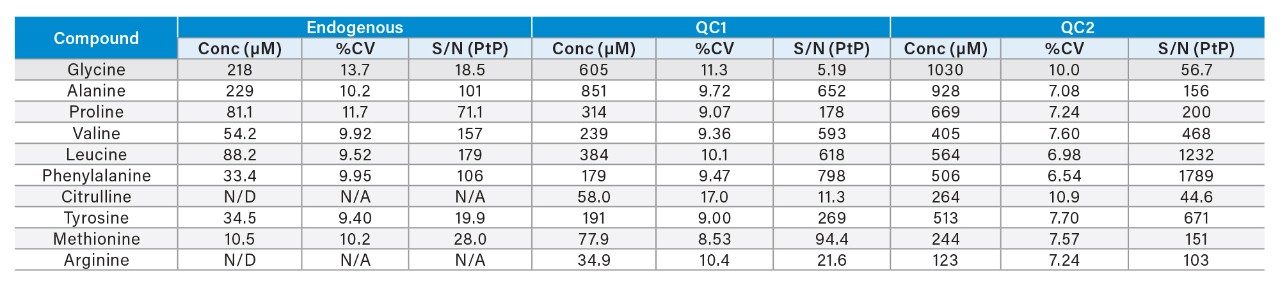
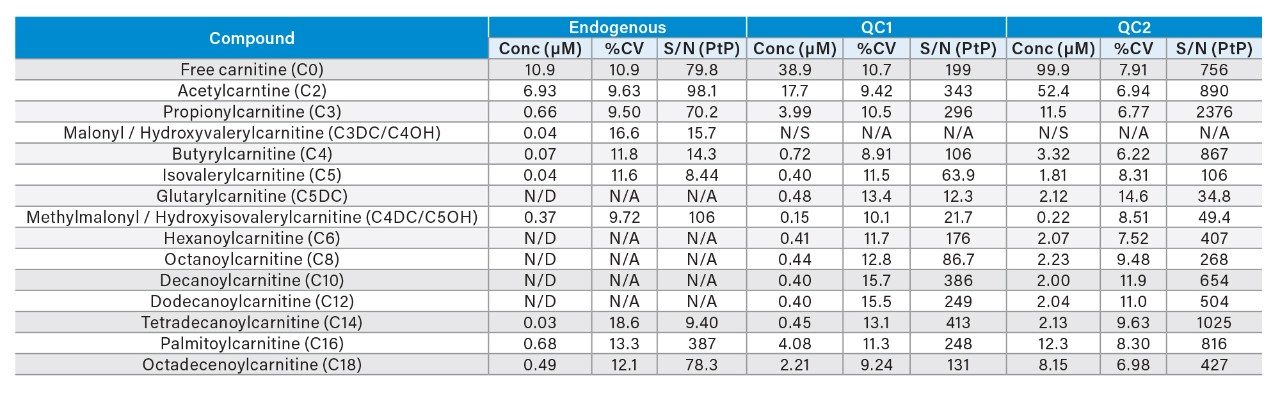
The imprecision of extraction and analysis of amino acids and acylcarnitines is illustrated in Tables 1 and 2. The Peak-to-Peak (PtP) Signal-to-Noise ratio (S/N) is shown, as an indication of the analytical sensitivity of the system.
Conclusion
The Waters RenataDX Screening System has demonstrated the capability to measure a subset of amino acids, free carnitines, and acylcarnitines. The endogenous concentration of some analytes was at the limit of detection of the RenataDX System.
Disclaimer
The analytical performance data presented here is for illustrative purposes only. Waters does not recommend or suggest analysis of the analytes described herein. These data are intended solely to demonstrate the performance capabilities of the system for analytes representative of those commonly analyzed using flow-injection analysis and tandem mass spectrometry. Performance in an individual laboratory may differ due to a number of factors, including laboratory methods, materials used, intra-operator technique, and system conditions. This document does not constitute a warranty of merchantability or fitness for any particular purpose, express or implied, including for the testing of the analytes in this analysis.
Featured Products
720006349, July 2021