This work represents a fast, simple, and elegant solution to this problem through the use of Oasis mixed-mode cation exchange (MCX) SPE. Basic analytes are bound to the sorbent by strong cation exchange, while non-ionic excipients such as Triton bind by reverse phase, and can be selectively removed before analyte elution.
Pharmaceutical dosing vehicle excipients such as Triton X-100 are often added to formulations to facilitate dissolution in dosing media. Unfortunately, these compounds can cause significant matrix effects, typically ion suppression, in LC-MS/MS analyses. The use of fast LC gradients, common in drug discovery, can result in co-elution of target analytes with these compounds. Early pharmacokinetic (PK) time points, when the concentration of these excipients is elevated, can be particularly troublesome as this co-elution has been shown to be a major contributing factor to excipient related ion suppression.1-3 Several approaches have been investigated to attempt to minimize this problem, including LC gradient manipulation,2,3 alternative analytical column choices,1 different sample preparation strategies,1-3 sample dilution,5 and even the development of a novel formulating agent.4 While some of these strategies have been successful, they each have their limitations. Clearly, the choice of formulation excipient is often beyond the control of the analyst. LC optimization, either by gradient or mobile phase manipulation, or by alternative column selection can solve this problem for some analytes, but not always, and requires additional method development. In addition, extensive manipulation of LC conditions is not always conducive to a high-throughput screening environment.
This work represents a fast, simple, and elegant solution to this problem through the use of Oasis mixed-mode cation exchange (MCX) SPE. Basic analytes are bound to the sorbent by strong cation exchange, while non-ionic excipients such as Triton bind by reverse phase, and can be selectively removed before analyte elution.
A basic drug mixture was prepared that consisted of acebutolol, doxylamine, labetolol, metoprolol, midazolam, and pindolol. This was either added to plasma samples prior to extraction to achieve plasma concentrations of 200 ng/mL or used to post spike blank extracted plasma eluates for recovery calculations. Triton X-100 was either added to plasma prior to extraction at a concentration of 5 mg/mL or used to post-spike extracted blank plasma eluates for determination of % removal. Triton X-100 was also used to post-spike extracted plasma samples to determine matrix factors associated with this excipient.
Samples were extracted according to the basic Protocol for Oasis MCX. Briefly, 0.5 mL of rabbit plasma was acidified with 0.5 mL of 4% H3PO4. (Note: 0.5 mL of plasma was used in this study; smaller volumes may also be used). After conditioning MCX plates with 1.0 mL MeOH and 1.0 mL H2O, acidified samples were loaded onto the sorbent bed. Loaded wells were then washed with 1.0 mL of 2% formic acid, followed by 2 x 1.0 mL of MeOH. The samples were then eluted with 2 x 250 µL of MeOH containing 5% NH4OH. “Post spike” (PS) samples were prepared by extracting blank rabbit plasma and post-spiking the final eluate with either the basic drug mix or a combination of the basic drug mix and Triton X-100. “Extracted” samples were prepared by adding either 200 ng/mL basic drug mix or 200 ng/mL basic drug mix + 5 mg/mL Triton X-100 to plasma prior to extraction.
Drug recovery and determination of Triton removal were determined by the following equation:
% Recovery = (peak area in extracted samples/peak area in post-spike samples) x 100%
For analysis of Triton X-100 removal, samples were diluted 1:20 in mobile phase to avoid contaminating the mass spectrometer with high concentrations of Triton X-100.
|
SPE Plate: |
Oasis MCX 96-well plate 30 µm (30 mg) |
|
Part Number: |
186000248 |
|
LC System: |
Waters ACQUITY UPLC system |
|
Detection: |
Waters SQD |
|
Vials: |
2 mL 96 well collection plate |
|
Part Number: |
WAT058958 |
|
Column: |
ACQUITY UPLC BEH C18 1.7 μm, 2.1 x 50 mm column |
|
Part Number: |
186002350 |
|
Column Temp.: |
30 °C |
|
Sample Temp.: |
10 °C |
|
Injection Volume: |
10 μl |
|
Flow Rate: |
0.5 mL/min. |
|
Mobile Phase A (MPA): |
0.1% HCOOH |
|
Mobile Phase B (MPB): |
Acetonitrile containing 0.1% HCOOH |
The initial mobile phase conditions were 95:5 MPA:MPB. Following a 0.5 min hold, MPB was increased to 95% over 2.5 min. and held for 1 min. The percentage of MPB was then returned to 5% and held for 2 minutes to re-equilibrate the column. The total run time was 6.0 min.
|
MS System: |
Waters SQD |
|
Ionization Mode: |
ESI Positive |
|
Acquisition Range: |
SIR |
|
Capillary Voltage: |
2.5 kV |
|
Cone Voltage: |
Compound specific (optimized for each analyte) |
|
Desolvation Gas: |
700 L/min. |
|
Cone Gas: |
0 L/min. |
|
Chromatography: |
MassLynx v4.1 SCN714 and MS Software |
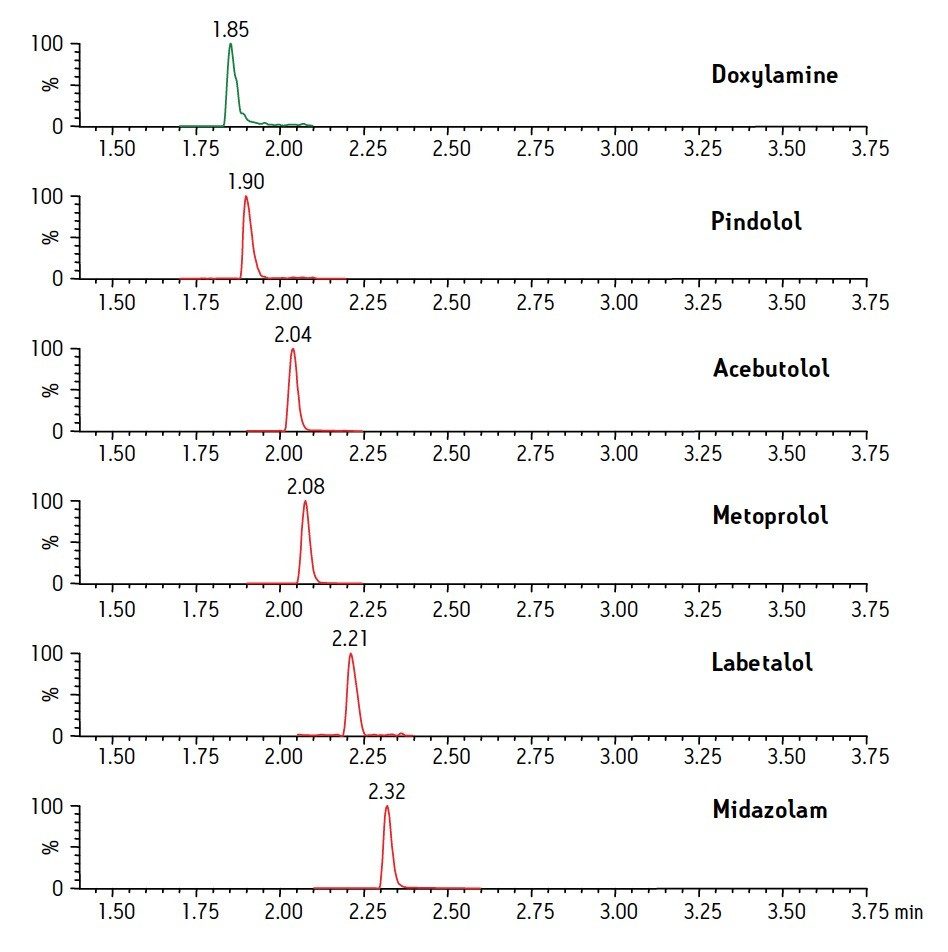
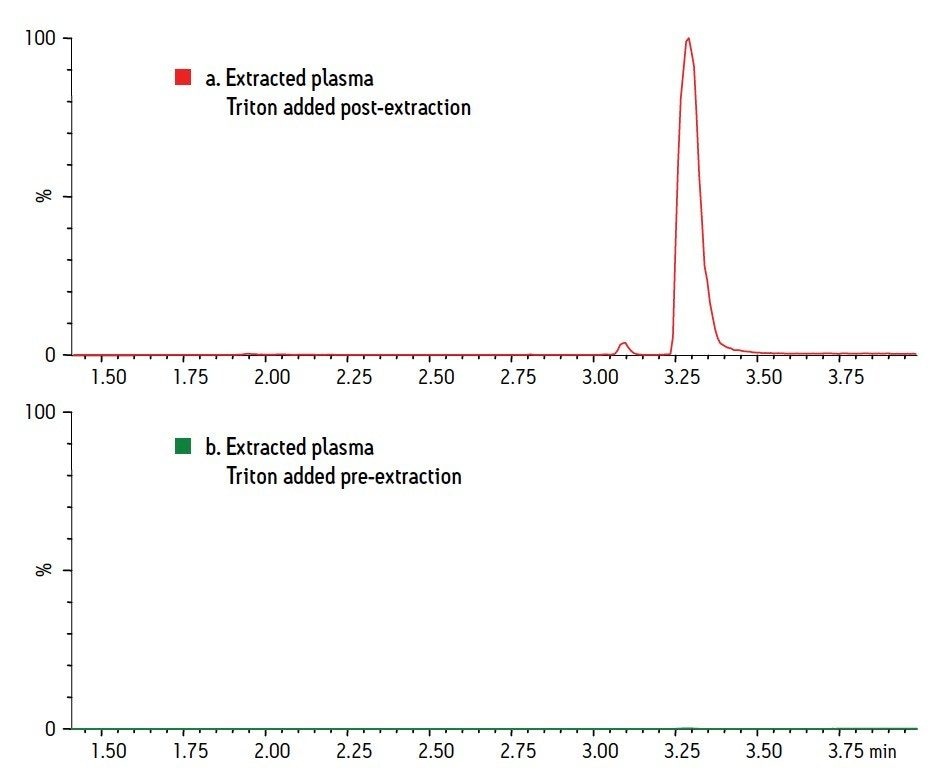
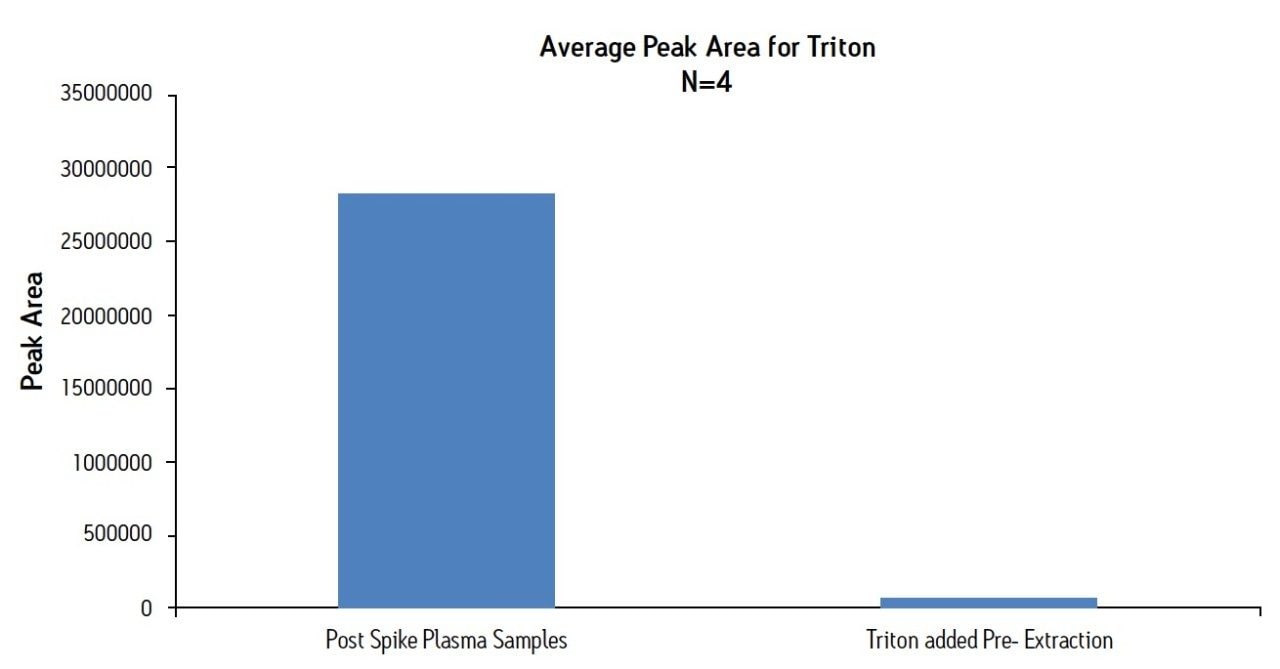
Figure 1 shows the chromatography of the 6 test compounds. These analytes (acebutolol, doxylamine, labetolol, metoprolol, midazolam, and pindolol) are represented by SIR traces of the MH+ ion, with the exception of midazolam, in which the MH+ is combined with (M-35)H+. Figure 2 shows two chromatograms of Triton X-100. The peak for Triton X-100 actually represents the combined TICs of the molecular ions from the most abundant components of Triton. This includes individual polymers with masses of 531, 575, 619, 663, 707, 751, and 795. Panel A indicates the level of Triton that would be present in an extracted plasma sample without removal by SPE. It was prepared by spiking Triton into the final plasma eluate post-extraction. Panel B is a chromatogram from a plasma sample containing 5 mg/mL Triton that was extracted using the standard MCX protocol, and demonstrates the nearly complete removal of Triton from the plasma sample. Figure 3 shows the average Triton peak area from each sample group in the experiment and demonstrates that 99% of the Triton was removed from plasma by the MCX extraction procedure.
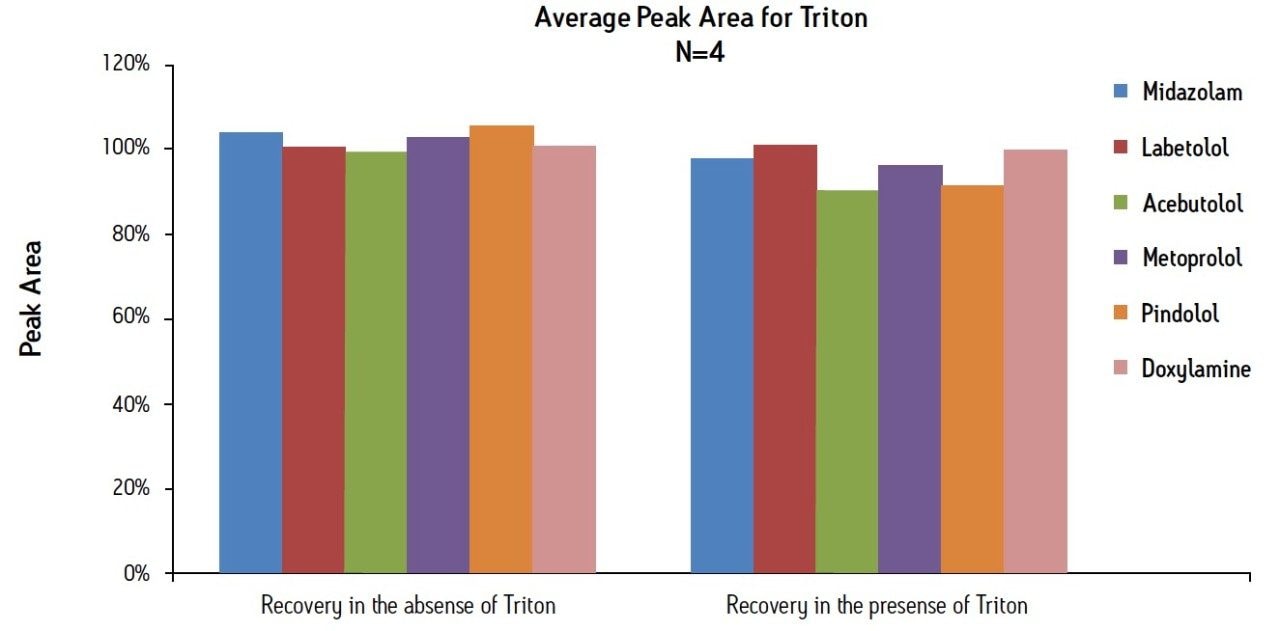
Analyte recovery was calculated according to the formula described in the methods section. Figure 4 shows that all analytes had recoveries that ranged between 99 and 105% using the basic MCX protocol. The average analyte extraction recovery was 102% for the six analytes. The presence of 5 mg/mL Triton X-100 in the plasma did not affect analyte recovery. The average recovery in the presence of Triton was 96%.
One of the key goals of Triton X-100 removal from plasma is the elimination of excipient related ion suppression. Matrix factors were calculated according to the equation below, as described in a recent AAPS article,6 and found to be close to 1 for all analytes (0.9-1.01; mean = 0.96) in the presence of Triton. This is most likely due to the fact that none of the analytes co-elute with Triton in this method, as matrix effects from dosing excipients generally occur during the elution of the excipient.1-3
Matrix factor = (peak response in presence of Triton/peak response in absence of Triton)
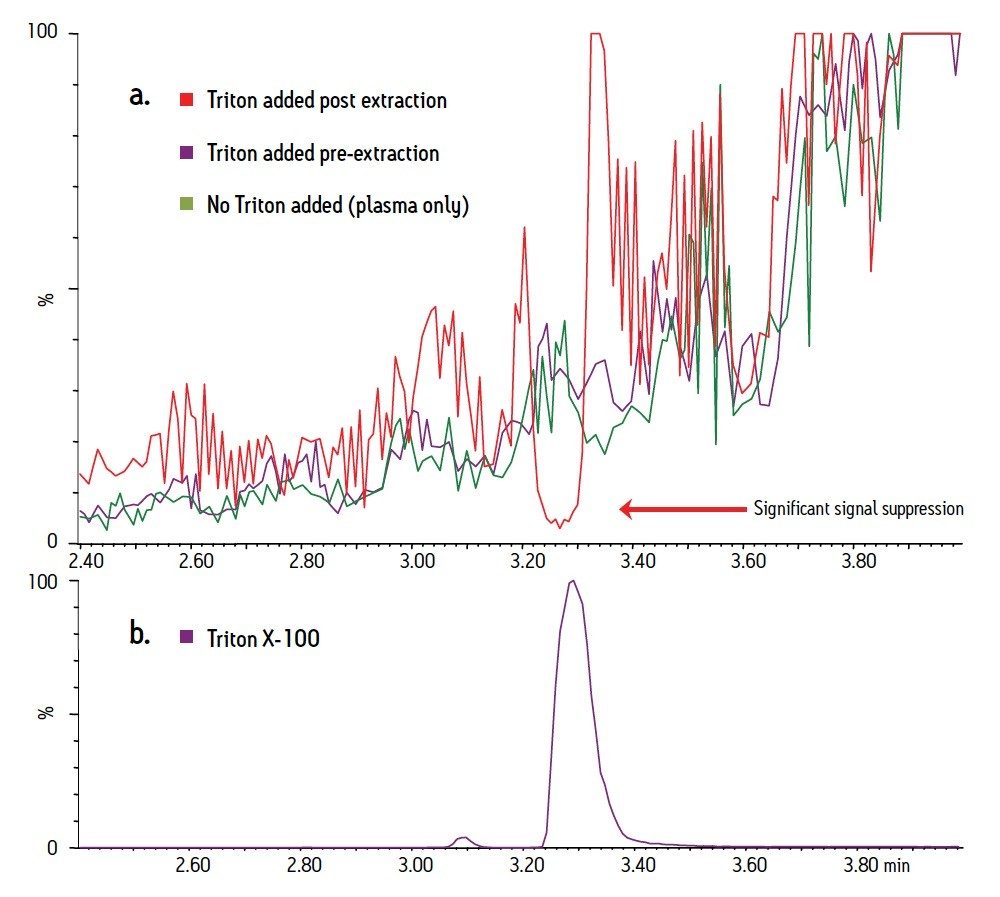
Therefore, in order to determine the expected impact of matrix effects if analytes were to co-elute with Triton, it was necessary to conduct a post-column infusion study. Briefly, the drug mixture was co-infused with the column effluent into the MS source. By comparing the target ion traces of the individual compounds, we were able to assess the degree to which Triton X-100 affected the signal intensity of the analytes. An example of these experiments is shown in Figure 5. Panel A shows three traces of the MH+ ion for pindolol, at a concentration of 500 ng/mL, infused at 10 μL/min with the column effluent. The green trace is from a plasma sample in which the drug mixture was added before extraction. The purple trace is from a plasma sample in which the drug mixture and 5 mg/mL Triton X-100 were added before extraction. The red trace is from a sample in which Triton X-100 was post-spiked into the sample eluent after extraction. Panel B shows the chromatography of Triton X-100 in the post-spike sample for comparison. This figure shows if Triton is not removed from the sample, it would result in a signal reduction of 80-90% for pindololol (red trace). This suppression is eliminated when Triton is removed by MCX (purple trace). The other analytes showed similar results. Triton associated ion suppression ranged between 50-85% and was eliminated after MCX extraction. Most importantly, it complements the data from Figures 2 and 3 and shows that MCX not only removes 99% of the Triton from plasma samples, but completely eliminates the ion suppression caused when Triton is not removed.
Waters Oasis MCX extraction plates represent a simple, quick and elegant method for the removal of high concentrations of Triton X-100 from plasma samples, eliminating matrix effects (and thus ion suppression) often seen in early time points of pharmacokinetic studies. Furthermore, this problem is solved without any additional chromatographic method development, sample dilution or other time consuming or cumbersome procedures, which makes it suitable and broadly applicable for a high-throughput environment.
720004157, October 2011