
For forensic toxicology use only.
This application note demonstrated the automated and fast method development capability of the ACQUITY UPLC System with 2D Technology for the analysis of cocaine and its metabolites in rat bone sample.
In the field of forensic toxicology, several challenges exist with quantification analysis of cocaine in post mortem samples, including its rapid half-life due to hydrolysis within hours of death and postmortem redistribution. Cocaine can prove difficult to quantify in blood, urine, and soft tissues and correlate findings with drug dosage before death. Alternative matrices, such as hair, nails, and bone could prove useful in detecting chronic drug use in postmortem toxicology cases. If a human body has undergone decomposition and putrefaction, toxicology screens of soft tissue samples are difficult to accomplish as well.
Detection and quantification of drugs in complex matrices is difficult to accomplish due to time-consuming extraction processes, and inability to detect an analyte at trace levels. Further, analysis of drugs in hard tissues, such as hair and bone, has only been attempted in recent years. Even fewer studies have investigated detection of drugs following decomposition of remains, specifically outdoor decomposition. A robust extraction and clean up methodology, in which a homogenization step precedes, is required to efficiently extract drugs from complex matrices, reach a target limit of detection (LOD) and to maintain instrument performance. The use of advanced hyphenated instrumentation platforms, such as UPLC-MS/MS has allowed analysts to detect trace levels of analytes. However, there is a delay in analysis due to the out-dated, extensive and time-consuming sample preparation protocols required to reach sub ng/mL levels. Traditional solid phase extraction techniques used in most laboratories require a lengthy evaporation step, which can take hours. A micro extraction protocol combined with a multi-dimension chromatography can decrease sample preparation time without sacrificing the quality seen with current single dimension chromatography techniques.1,2,3,4
Two MRM transitions (quantification and confirmation) for Cocaine, Benzoylecgonine, and Ecgonine methyl ester were selected and optimized. The MRM conditions are listed in Table 1.
All rat specimens used for this study underwent 10–12 week chronic intravenous self-administration of cocaine. This was followed by a six-week period of abstinence, followed again by a three-week period of cocaine self-administration before being euthanized. Average daily dosages for each rat fell within a range of 13–19 mg/kg. Fourteen cocaine positive rats were placed outside and above ground in a gated facility for a period of 12 months. All recoverable skeletal samples were collected for testing. Drug free control rat bones were also acquired by placing drug-free rats outdoors, above ground, until full decomposition occurred.
For this application, finding the optimum extraction and chromatographic condition for this multi-residue analysis posed a significant challenge. As seen in Figure 1, Cocaine, Benzoylecgonine, and Ecgonine methyl ester share a common rigid aromatic structure. The chromatographic conditions were tested on several trapping chemistries (Oasis HLB, XBridge C18 and XBridge C8) and separation chemistries (BEH C18). The loading (low pH, high pH & neutral pH) and eluting mobile phase (MeOH + 0.5% formic acid & ACN + 0.5% formic acid) were also optimized using an automated 6x6 process.

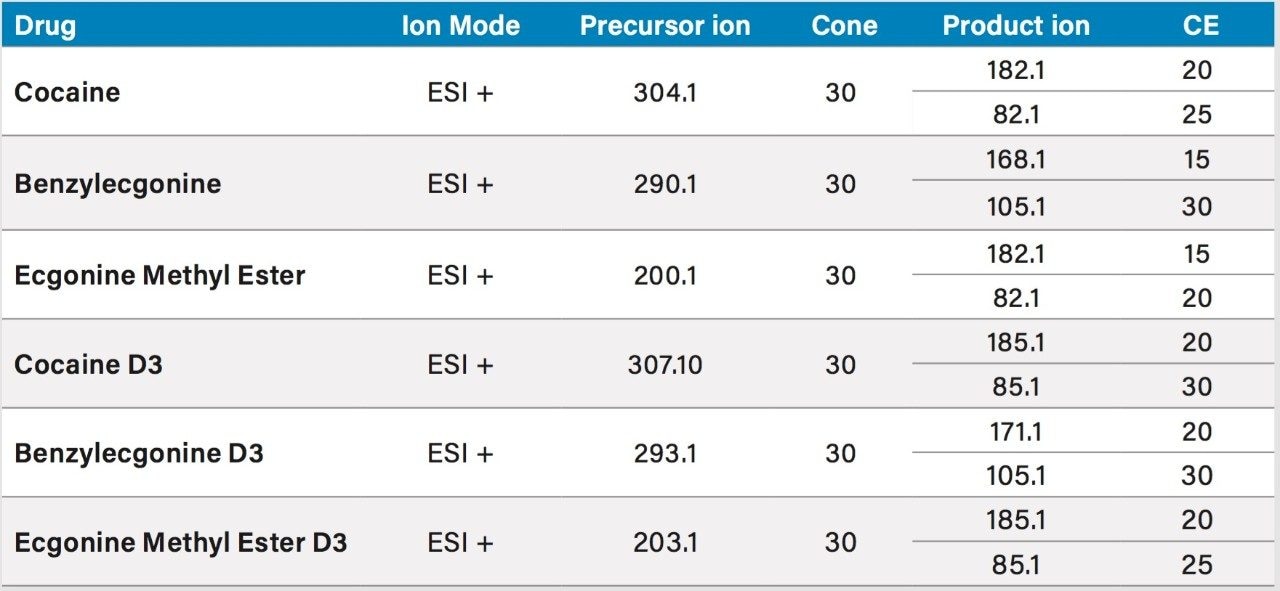
The homogenization process started by measuring 0.5 g of bone followed by an addition of 4 mL of methanol in a 15 mL plastic tube. The bone sample was homogenized using a high speed impact process of 6000 rpm with ceramic ball bearings for approximately 5 minutes. The methanol homogenate was centrifuged at 4000 rpm for 5 min and the supernatant collected for further extraction. The solid homogenate was re-extracted with 4 mL of water and the aqueous supernatant was pooled with the previous method extract for the next step.
The pooled extracts were then filtered using a 0.45 μm size PTFE filter. The final filtered extract was then diluted in 100 mL of MilliQ water. The extraction process was performed on pre-conditioned mixed mode reversed phase/ion exchange sorbent (Oasis MCX 6 cc SPE barrel). The mixed mode approach yields two eluting fractions, one fraction comprised of neutral and acidic entities and the other fraction concentrating the analytes with basic functionalities. The cartridge was washed with 2 mL water with 0.1 N HCl, followed by 2 mL of MeOH with 2% formic acid. The target analytes were eluted with 2 mL 100% MeOH with 5% ammonium hydroxide (See Figure 2). From an acetonitrile stock solution of 100 ppb, 20 μL of IS mix (Cocaine D3, Benzoylecgonine D3, Ecgonine methyl ester D3) was added to the 2 mL elution to attain a final internal standard (IS) concentration of 1 ppb. The use of a 2D LC-MS/MS technology eliminates the need for an evaporation step in the extraction method. The manual extraction and sample preparation of the bone samples was completed in less than one hour. The analysis was performed using 100 μL of the final organic solvent (MeOH) extracts.
|
Loading conditions |
|
|
Column: |
Oasis HLB 20 μm – 40 mg (3.9 x 5 mm) |
|
Loading: |
MilliQ Water (pH 10, 2% NH4OH) |
|
Flow rate: |
2 mL/min |
|
At-column dilution: |
5% (0.1 mL/min Loading pump and 2 mL/min Diluting pump) |
|
UPLC system: |
ACQUITY UPLC with 2D Technology configured for “Trap & Elute” with At-column dilution |
|
Runtime: |
10 min |
|
Column: |
ACQUITY UPLC HSS T3, 2.1 x 50 mm, 1.7 μm |
|
Column temp.: |
60 °C |
|
Mobile phase A: |
Water + 0.5% formic acid |
|
Mobile phase B: |
Acetonitrile + 0.5% formic acid |
|
Elution: |
5 minute linear gradient from 5% (B) to 95% (B) |
|
Flow rate: |
0.5 mL/min (Elution pump) |
|
Injection volume: |
100 μL |
|
MS system: |
Xevo TQD |
|
Ionization mode: |
ESI Positive |
|
Capillary voltage: |
3.0 kV |
|
Cone voltage: |
90.0 V |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
550 °C |
|
Desolvation gas: |
1100 L/hr |
|
Cone gas: |
50 L/hr |
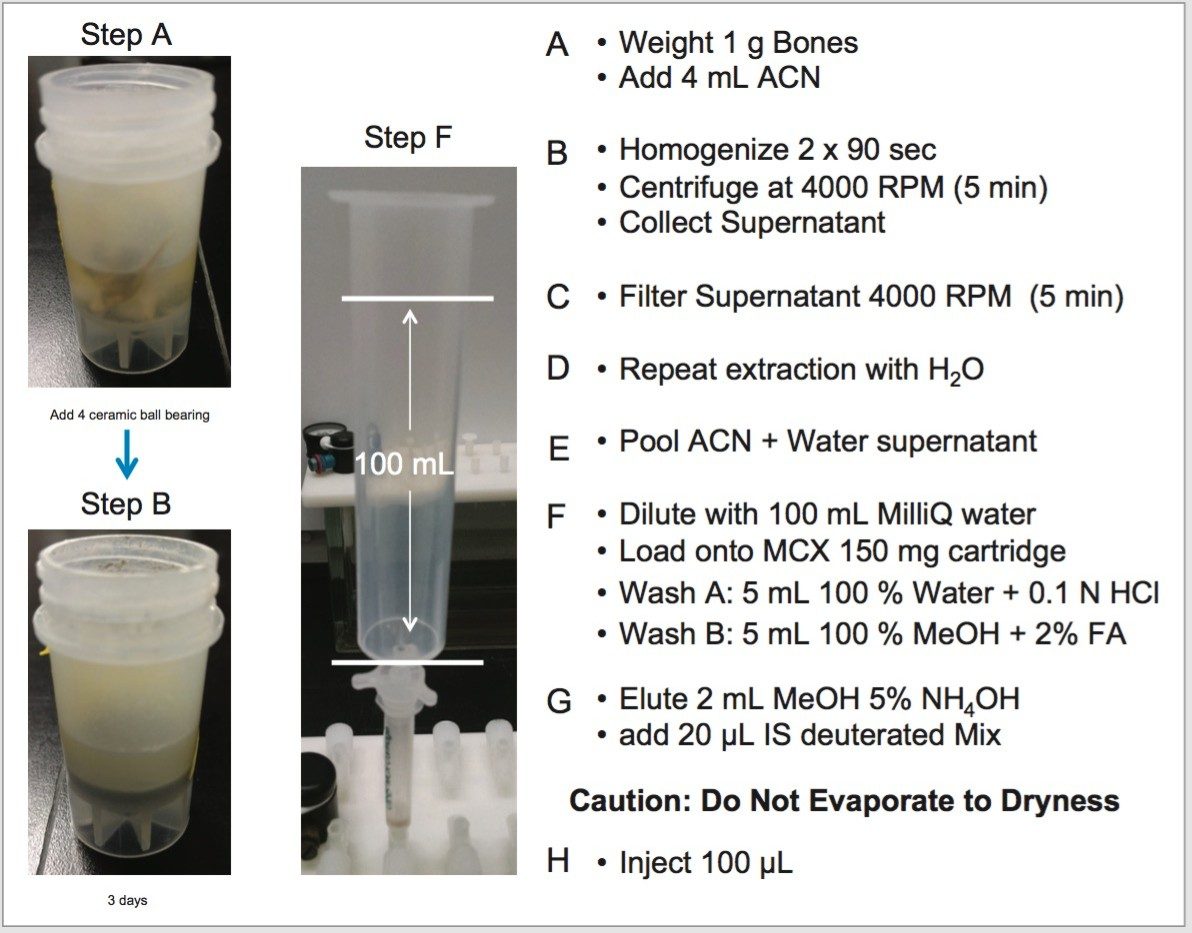
The analysis of cocaine, benzoylecgonine and ecgonine methyl ester started with the chromatography optimization of the 2D LC-MS/MS. The 2D LC-MS/MS is setup as depicted in Figure 3. This configuration was constructed with two binary pumps, one set for gradient elution and the second pump was re-plumbed for At-column dilution to create two streams.
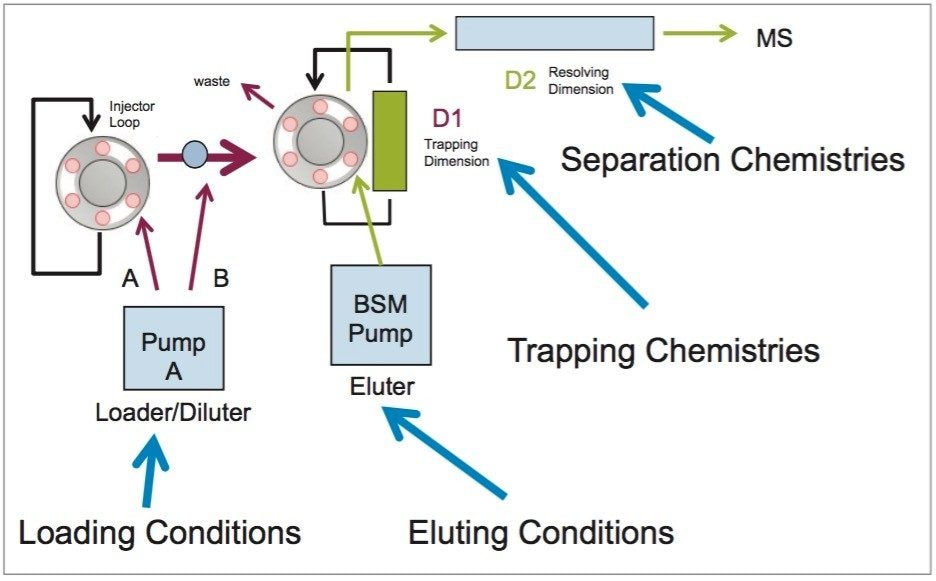
The A side was set for loading the extracts from the injection loop onto a 50 μL mixer, while the B side was set at high flow rate for dilution following a re-focusing effect on the trap column. From the chemical structures of the target analytes, a high retention strength sorbent material (Oasis HLB) was selected for the trap column, while a high strength silica C18 sorbent (HSS T3) was chosen for the analytical column. The next phase of the optimization was to select the trapping and elution conditions. As seen in previous publications, a 6x6 2D LC evaluation grid (see Figure 4) gives an excellent starting point to provide an overview of the chromatographic behavior for a target analyte. For this application, the 2D LC optimization process focused with methods 3, 6, 9, 12, 15, and 18. The results are tabulated in Table 2.
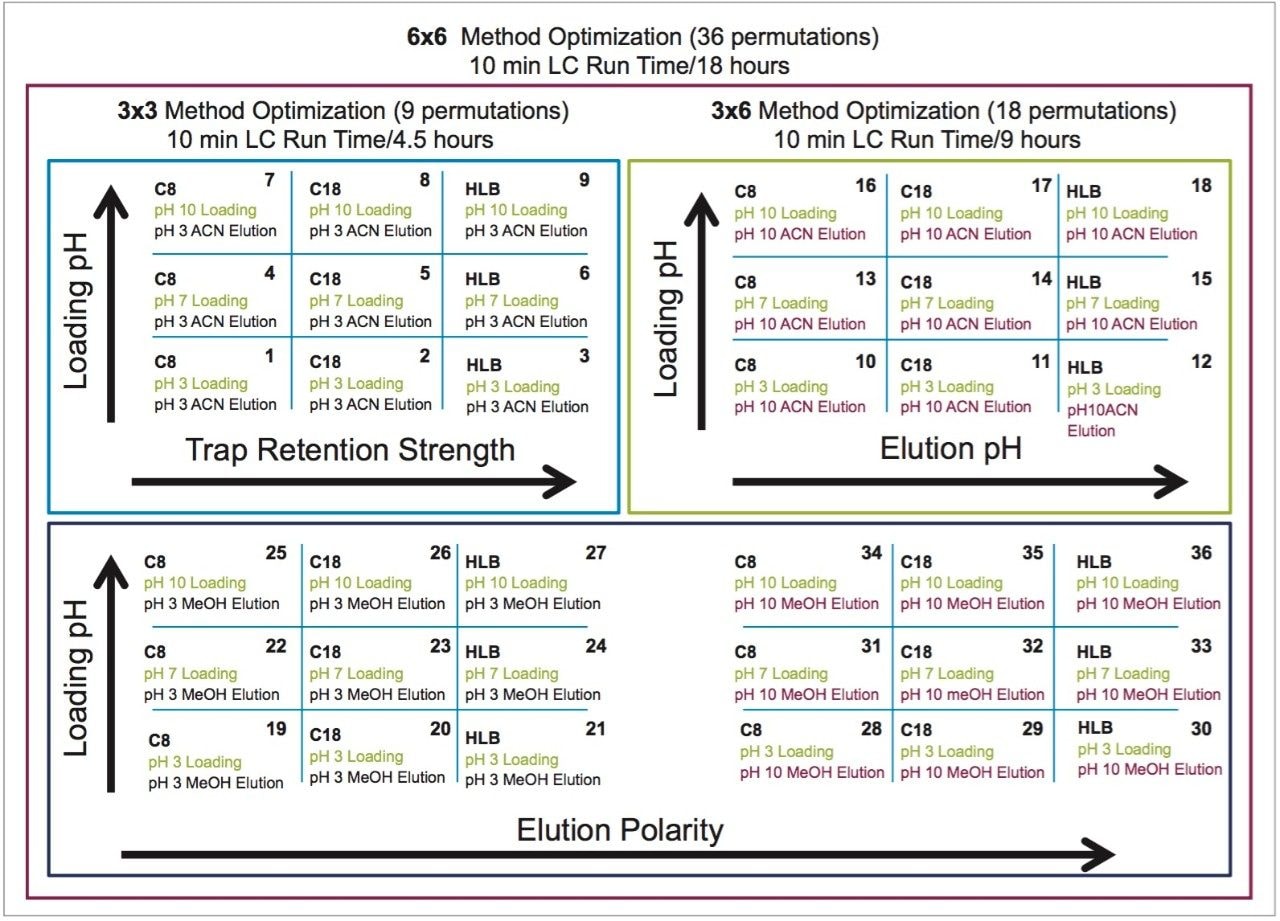
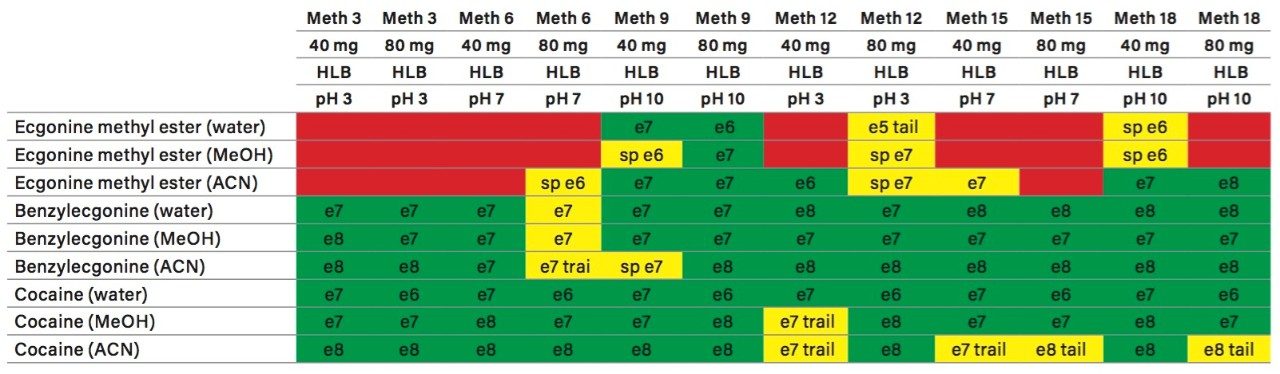
The color coded chart was created to identify which analytical conditions give the best chromatographic profile with a quick visual survey. The green box depicts a Gaussian peak shape for quantification analysis. The yellow box was used to flag chromatography issues, such as peak split, tailing, shoulder or leading profiles. Finally, the red box indicates an absence of signal, most likely due to breakthrough effect during loading phase on the trap column or poor elution from the trap onto the analytical column. Additional parameters can be adjusted to ensure proper mass transfer during loading and elution phase. One parameter in particular is the sorbent bed mass on the first dimension. Two sorbent bed masses (40 mg vs 80 mg) were evaluated for the retention and elution of the target analytes. As shown in Table 2, method 9 using an HLB 80 mg bed mass provided the best chromatography performance for Cocaine, Benzoylecgonine, and Ecgonine methyl ester. The conditions for method 9 showcases a high pH loading (pH 10) using a high retention trap sorbent, indicating that ecgonine methyl ester can only be trapped effectively as a neutral entity (pka 9.57) (see Figure 5).
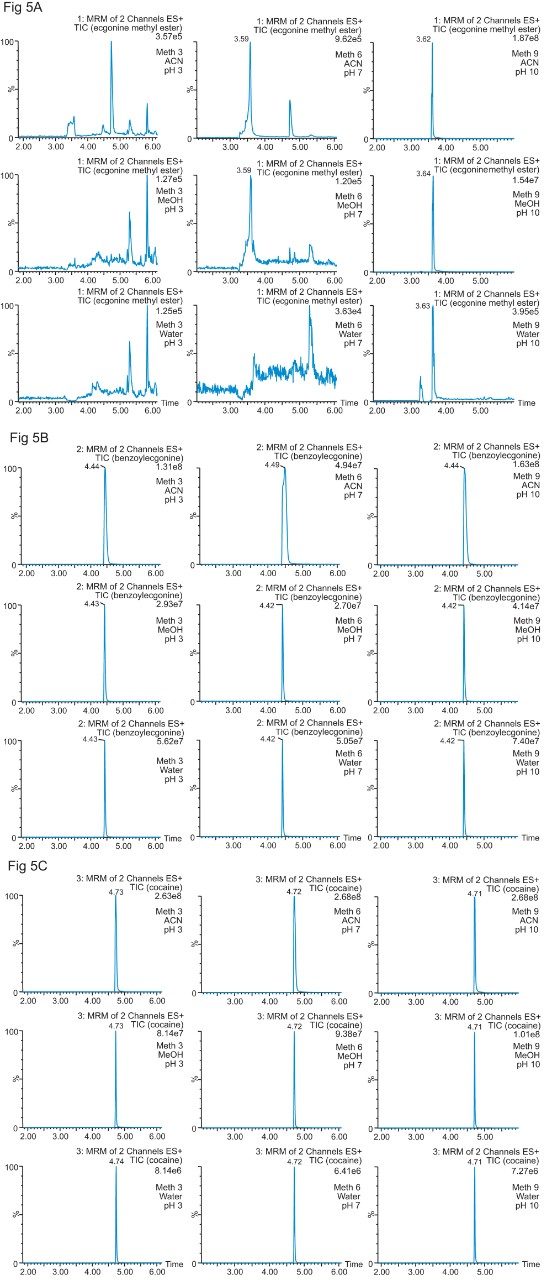
Therefore, the final protocol will use a pH 10 loading onto an 80 mg HLB on the first dimension, followed by an elution with acetonitrile at pH 3 onto a HSS T3 analytical column. The final separation showed excellent Gaussian peak shapes for all three analyte. However, for a water spike, lower intensities are usually expected due to secondary interactions with the active sites, most likely due to ion exchange retention with the glass vial surface. The ionic interaction can be eliminated by simply changing the diluent. In this case, methanol and acetonitrile diluents both gave higher intensities.
After selecting the optimum 2D LC conditions, the bulk of the work focused on the extraction optimization for cocaine, benzoylecgonine and ecgonine methyl ester. Since all three analytes contain a nitrogen atom in their distinct structures, it was expected that these molecules would exhibit high pKa values. As such, cocaine is a weak base with a pKa of 8.6, ecgonine methyl ester has a strong pKa value of 9.57, however, benzoylecgonine displays two stronger pKa's, one acidic at 3.35 and one basic at 10.82 value.
In this application, since the target matrix is very high on the complexity scale (Class C matrix), the extraction protocol will require a robust clean up methodology and an evaluation of optimum extraction conditions during the homogenization process. Therefore, the first step was to choose a solid phase extraction (SPE) protocol with a superior clean up capability. In Figure 6, a mixed mode sorbent (Reversed-Phase/Cation- Exchange) was evaluated for the retention and elution of the three target analytes spiked at 1 ppb in 100 mL water. The results are listed in Table 3. For maximum retention, two bed masses were evaluated. The bed mass sizes included a 60 mg (3 cc volume capacity) and 150 mg (6 cc volume capacity). With the 60 mg and 150 mg cartridge, the extraction values for cocaine showed consistent levels for all six elution solvents, while only the methanol elution on the 150 mg bed mass demonstrated acceptable responses for benzoylecgonine and ecgonine methyl ester.
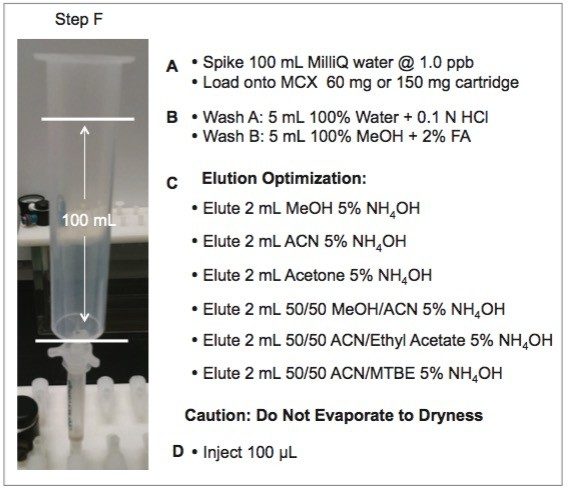

The next phase of the application was to optimize the solid-liquid extraction of the solid sample (rat bones) and evaluate the proper loading condition onto the mixed mode SPE sorbent. The homogenization process is typically performed with a common kitchen blender or a hand-held homogenizer (ex: Polytron). Those techniques can be cumbersome and are difficult to apply to small mass samples. In recent years, novel developments with ceramic or stainless steel ball bearings in combination with high speed orbital shakers have shown the ability to reach complete cell membrane breakdown in less than 60 seconds (see Figure 7). With variable cycle speed, this novel homogenization protocol can process sample sizes from 0.1 to 5 grams. In this application, the mass range of rat bone sample was between 0.2 and 0.8 grams. Once a sample was completely homogenized, it was centrifuged into a solid pellet on the bottom of the tube with the organic supernatant above. The organic supernatant was then filtered and decanted. Depending on the extraction conditions (pH and polarity), the target analyte is expected to be in solution and un-bound in the extraction solvent. In some applications, this crude extract can be used directly for quantification, however there is a high risk the raw sample extract will seriously reduce the robustness of the LC-MS/MS performance after a few injections. In traditional SPE protocols, when the target analyte is dissolved in a high percentage of organic solvent, the supernatant is usually evaporated to dryness and reconstituted in an aqueous diluent for further clean up. In instances where an evaporation-todryness step is needed, there is a risk of evaporative loss or possible re-dissolution issues. An effective way to avoid this lengthy step is to simply dilute the organic supernatant in a large aqueous volume at an organic/water ratio of less than 5%. A water volume between 100 and 200 mL is more than adequate to reach low organic ratio without any risk of breakthrough on the trapping column during loading phase. It may be perceived as a drawback, since the loading volume is quite large. However, with a loading flow rate at 10 mL/min and using a large bore SPE barrel (6 cc with 150 mg bed mass), a 100 mL sample can be concentrated in 10 min, while evaporating to dryness can take several hours to complete.

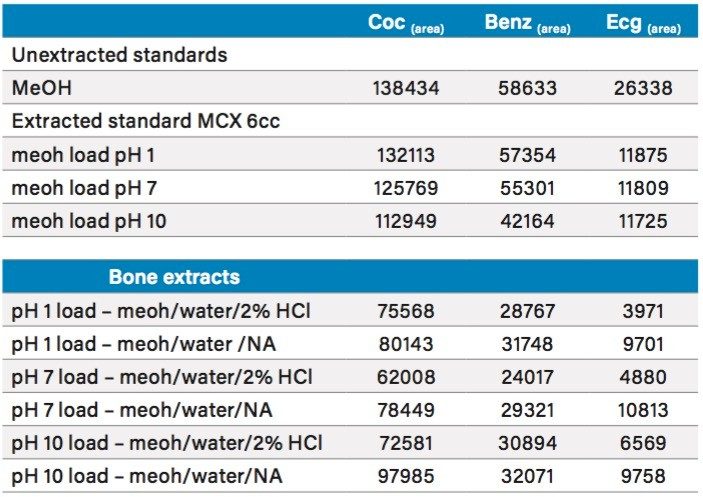
For this application, the bone samples were extracted with two solvents using two sequential steps. Preliminary results indicated that a bone sample extracted with methanol, followed by a second extraction with water would yield better results for the two metabolites, which appear to have a higher affinity for aqueous interaction than with polar solvents. For this portion of the work, a 0.5 gram bone sample was used to optimize the solid liquid extraction pH and loading pH onto the Oasis MCX cartridge. The results revealed consistent values for cocaine regardless of pH during extraction and SPE loading. The challenge was to find the optimum condition for the two metabolites. The extraction using methanol and water with no additive combined with a high pH loading gave the best recoveries for all three analytes (See Table 4).
With the extraction protocol finalized, the chromatograms in Figure 8 show the chromatography profile for a methanol standard, water extracted standard and a spiked bone sample at 500 ppt level. It is worth mentioning the stable baseline and the presence of one single interference in the ecgonine methyl ester trace in both the water and bone extract. This suggests that the extraction protocol is producing a very clean extract with an extraction time of 45 minutes.
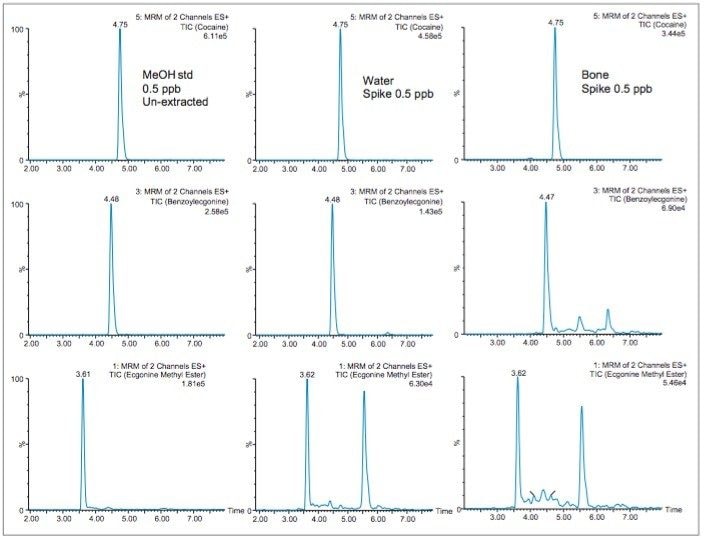
When analyzing highly complex sample types (class C matrix or solids samples), the extraction recoveries are most often overwhelmed by matrix effects, which can lead to either suppression or enhancement in the MS detector. These effects are related to the inability of the extraction protocol to remove interferences from the raw sample. In this work, the extraction protocol relies on a dilution effect (50:1) to avoid the time consuming evaporation to dryness. With the solvent exchange step eliminated, the organic extract from the homogenization process can simply be diluted to reduce the organic content below 5% (optimum value for loading without breakthrough effect during the trapping phase). However, large volume loading will lead to an enrichment effect, which, if poor water quality is utilized, will lead to possible enrichment of additional sources of interferences. For this reason, optima grade water was used for the dilution step. In most cases with complex sample analysis, the use of a deuterated internal standard (post or pre spike) is an effective technique to leverage suppression or enhancement effect from the total recovery. Also, the use of matrix match standards versus neat standards, either extracted or un-extracted, can lead to successful quantification.
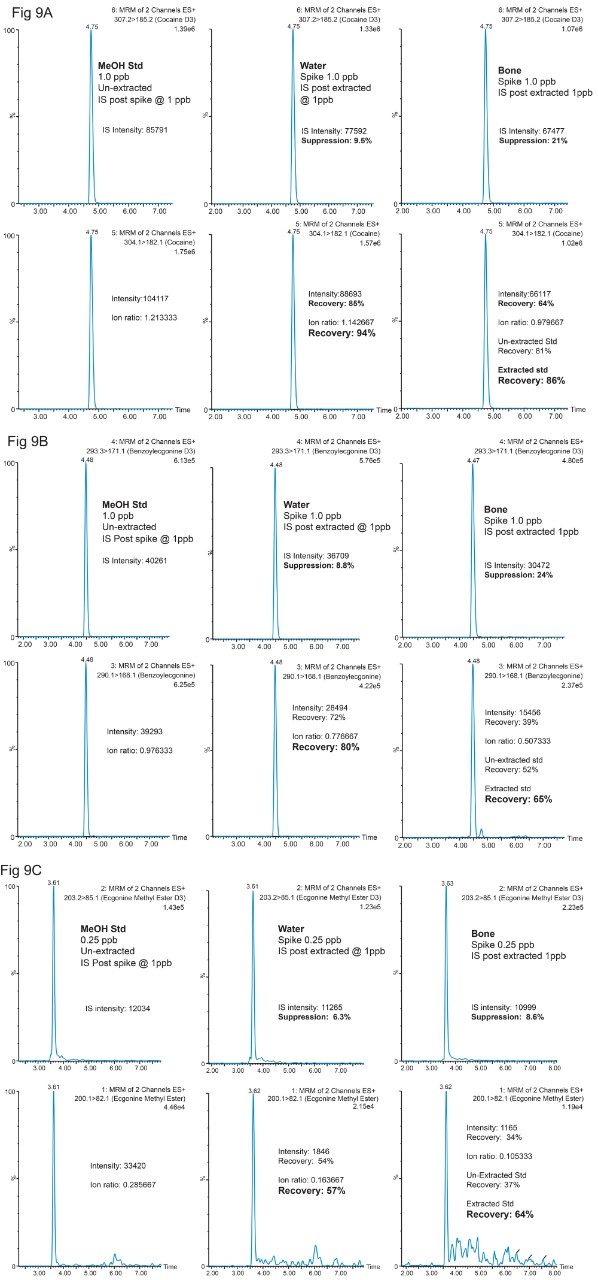
In figure 9A, 9B and 9C, several key chromatograms (neat std, water extract, bone extract) for Cocaine, Benzoylecgonine, Ecgonine methyl ester, showcase the matrix effects and recoveries between neat and extracted standards. For example, in Figure 9A for Cocaine, the bottom left chromatogram shows the response for a cocaine methanol standard at 1 ppb concentration. The deuterated D3 cocaine IS is displayed on the top left chromatogram. From this reference point, a water extracted standard is shown in the bottom middle chromatogram with the corresponding post-spike deuterated IS on top. By comparing the deuterated IS area counts from the neat standard and the post spike extracted standard, the suppression effect for the 100 mL MilliQ enrichment process was calculated at 9.5% for Cocaine, 8.8% for Benzoylecgonine and 6.3% for Ecgonine methyl ester. When using the area counts for recovery calculation for each target analyte, the results show an 85%, 72% and 54%, for Cocaine, Benzoylecgonine, and Ecgonine methyl ester, respectively. These recoveries include matrix effect and potential leachables/extractables from the MilliQ enrichment and the homogenization process. However, by using an ion ratio value (target analyte versus deuterated IS), the recovery value for the extracted water standard shows corrected values for matrix or method effects. Regarding bone analysis, the question concerning quantitation is a challenging one, can a neat extracted standard approach produce acceptable quantifiable results or will the complexity of the sample require a matrix match extracted standards for quantification. In this instance, the latter approach produced satisfactory recovery values in bone matrix of 86% for Cocaine, 65% for Benzoylecgonine and 64% for Ecgonine methyl ester.
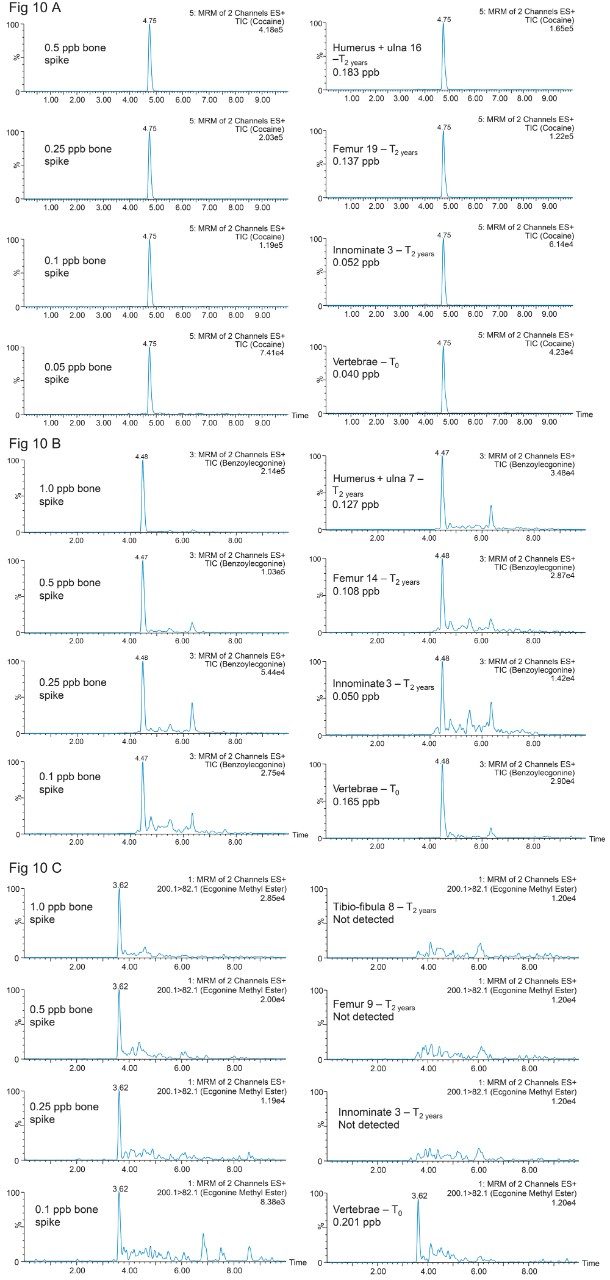
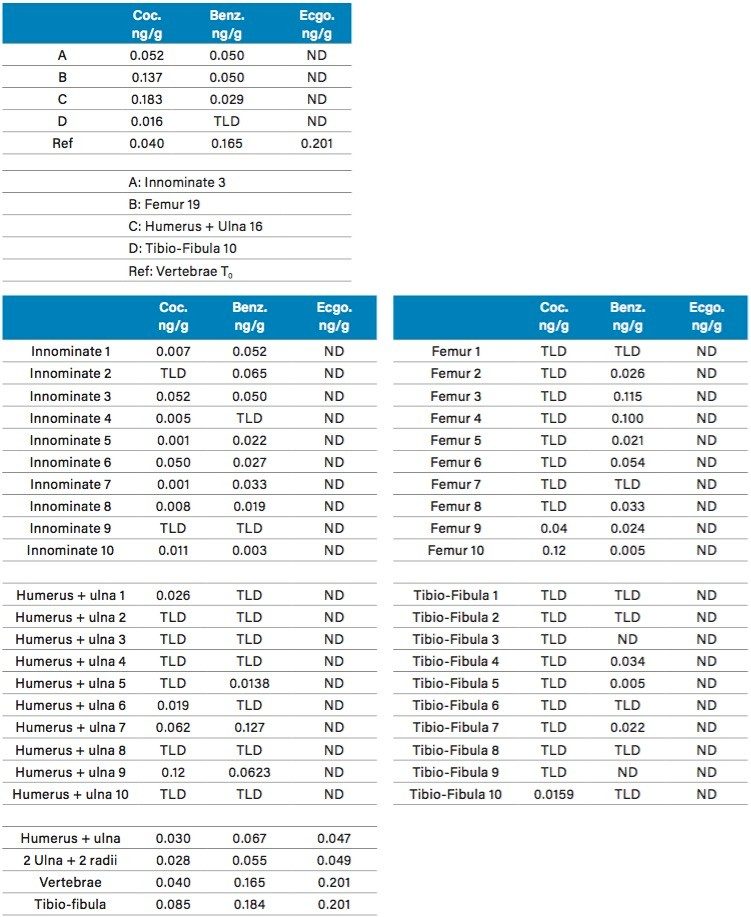
The calibration curve (0.05 to 10 ppb range) in bone extracts gave excellent linearity response with an R2 value of 0.998 for Cocaine. For the two Cocaine metabolites, the linear range was set from 0.1 to 10 ppb with a linear curve fitting and R2 values of 0.998 and 0.997 from Benzoylecgonine and Ecgonine methyl ester. The results for the biological specimens collected immediately after euthanizing, T0, (vertebrae, innominate, femur, ulna16, and Humerus) are listed in Table 5. Representative chromatograms for selected rat bones for cocaine, benzoylecgonine and ecgonine methyl ester are shown in Figure 10A, 10B, and 10C. The results are tabulated in Table 5. As seen, Cocaine and Benzoylecgonine can be detected in the 0.001 to 0.1 ng/g range. The chemistries used for this application gave an excellent performance analyzing well over 1000 sample injections.
This application demonstrated the automated and fast method development capability of the ACQUITY UPLC System with 2D Technology for the analysis of cocaine and its metabolites in rat bone sample. The quantification limit was set at 50 ppt using a 0.5 g of sample. The micro extraction protocol offered the option to evaluate several elution parameters in a short time period. The elution optimization was completed within a 4 hrs hands-on work and the 2D LC results were analyzed using an over-night run multi-methods sample list (18 hrs). With the extraction protocol optimized, the final protocol produced a clean extract in 40 minutes without any evaporation to dryness and reconstitution into initial mobile phase conditions. The reversed-phase/ion exchange extraction protocol gave an 85% recovery for cocaine.
720005847, December 2016