This is an Application Brief and does not contain a detailed Experimental section.
This application brief highlights the ease of implementation and efficient performance of N2 as a replacement carrier gas for helium (He) in GC separations interfaced with the atmospheric pressure gas chromatography (APGC) MS source.
Implementing nitrogen (N2) as the carrier gas for GC experiments is a cost-effective approach for atmospheric pressure MS sources, while maintaining critical separations and chromatographic performance.
Helium (He) is the most commonly used carrier gas in gas chromatography (GC) applications. However, the finite nature of reserves has resulted in periodic price increases and concern regarding availability.1 Nitrogen (N2) is a more affordable and readily available option that has historically been less utilized as a GC carrier gas. Reasons for this are that N2 has lower diffusivity than He or hydrogen and often requires longer run times to achieve similar separations. In this technology brief we show GC coupled with an atmospheric pressure ionization mass spectrometry, which utilizes N2 for both ionization and make-up flow. This allows a single gas source to be used for chromatographic separation as well as for ionization. Following automated method transfer calculations available within Waters UNIFI Software, efficient and comparable chromatography using N2 carrier gas was achieved for pesticides, polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), and chlorinated dioxin/furans (PCDD/Fs). Unlike electron ionization, atmospheric pressure ionization remains robust during the introduction of N2. Also, a higher range of column flows can be used in the APGC source compared to tradition vacuum GC, and more flexibility with regards to method translation such that optimized carrier gas linear velocities could be achieved for N2.
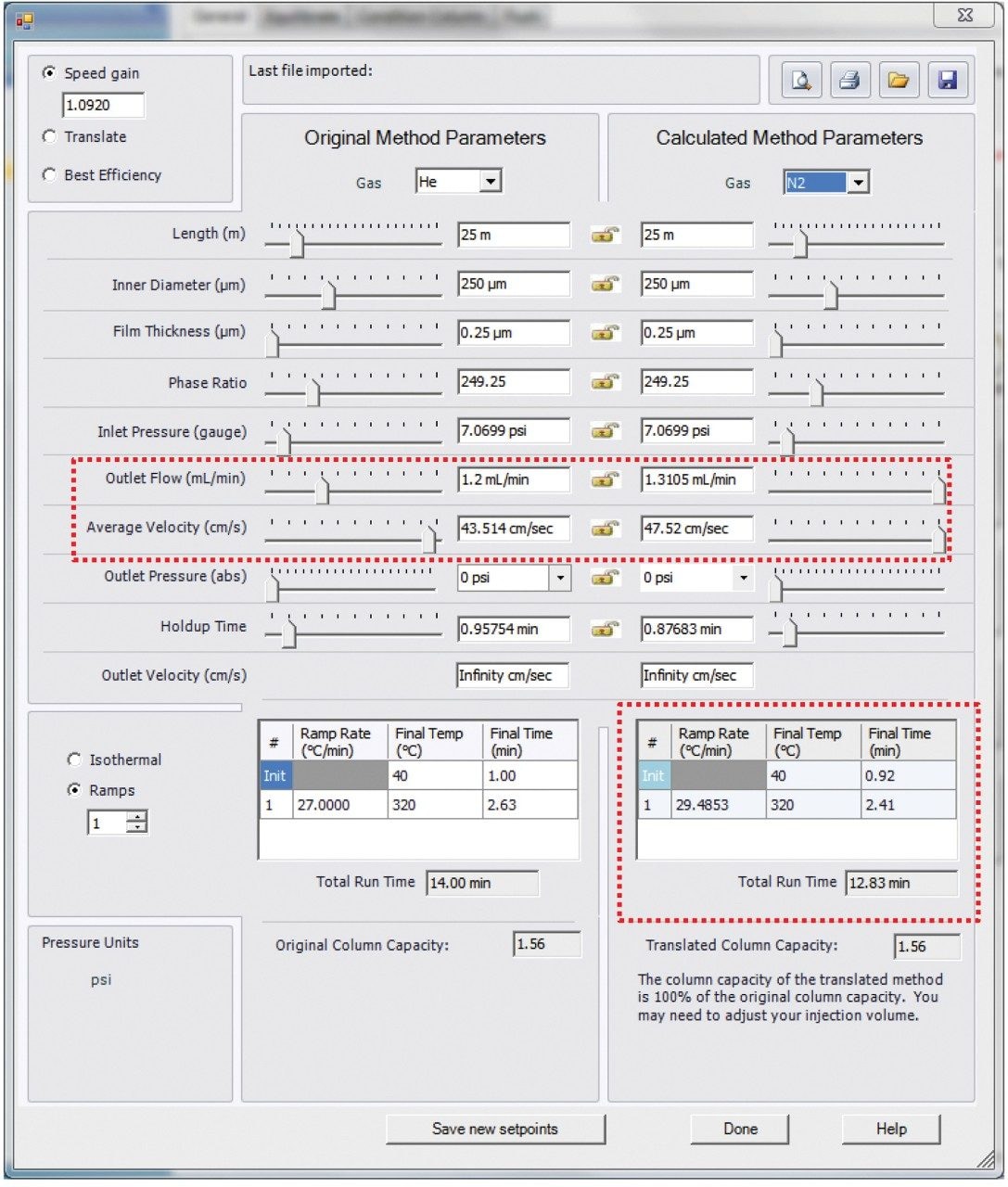
Experiments were performed on a Xevo G2-XS QTof with APGC (Figure 1). Ionization was performed using atmospheric pressure chemical ionization, such that protonation (resulting in the [M+H]+ ion) or charge transfer (resulting in the M•+ ion) reactions occurred. The GC method for pesticides is described in Table 1a and 1b, and for the analysis of PBDEs, PCBs, and PCDD/Fs in Table 2a and 2b. Methods were revised using an automated calculator for method transfer to arrive at optimum values for N2 as a carrier gas, resulting in comparable separations to those achieved using He. Figure 2 shows the calculator as available in Waters UNIFI Software for GC Instrument Control. When using the Speed Gain option of the calculator, the increase of the Outlet Flow rate (mL/min) and resulting Average Velocity (cm/s) resulted in a shortened method when N2 carrier gas was used as compared to He.
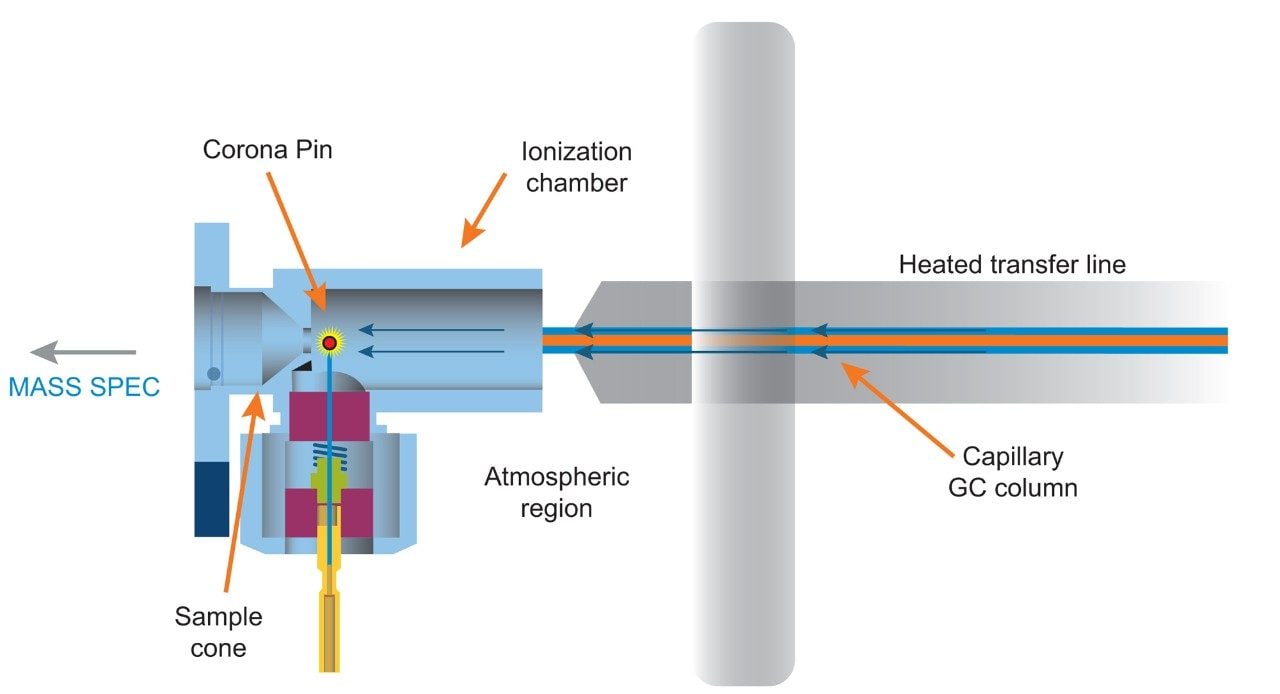
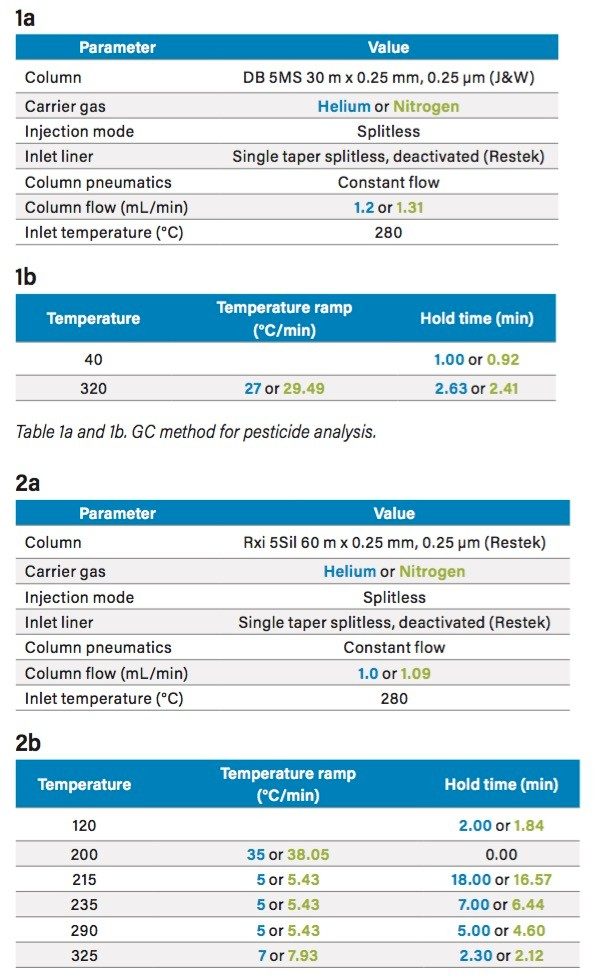
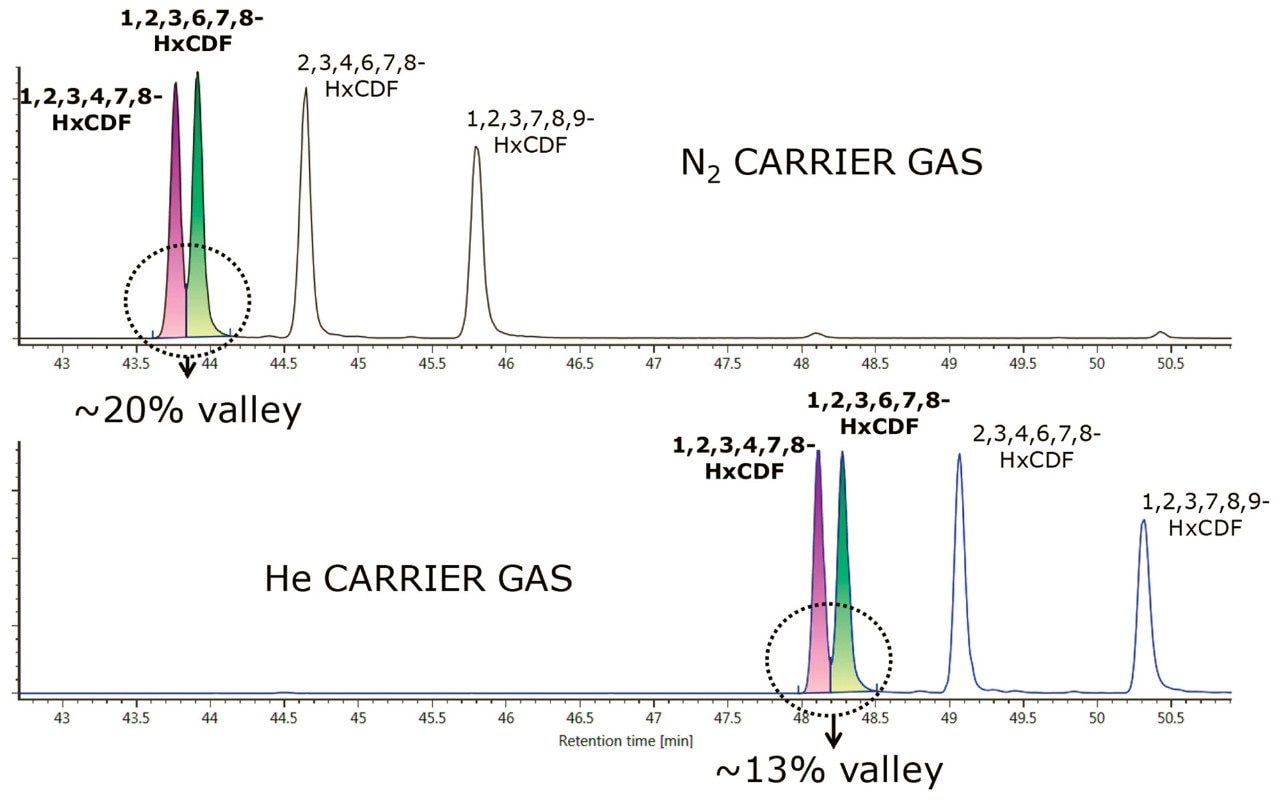
For the PBDEs, PCBs, and PCDD/Fs method, the separation of the closely eluting hexachlorodibenzo-p-furan (HxCDF) congeners 1,2,3,4,7,8– and 1,2,3,6,7,8-HxCDF is shown in Figure 3. A 25% valley is retained for the co-eluting 1,2,3,4,7,8– and 1,2,3,6,7,8-HxCDF congeners using both N2 and He separations, as specified in the EPA 1613 analytical guidance.2 In addition to both analytical assays retaining critical separations when using N2 carrier gas, a faster run time was achieved. The reduced lifetime of the GC filaments traditionally caused during electron ionization when using N2 carrier gas is eliminated by the use of an atmospheric pressure chemical ionization MS source. Thus far no negative implications from the use of N2 as a carrier gas have been observed or are expected.
Nitrogen can be used as a single gas source for both GC carrier and MS source gas flows using the APGC source, and presents a viable replacement option to Helium carrier gas. N2 optimized GC temperature program methods require less time without sacrificing critical separations for the pesticides and POPs analyses studied.
720005938, March 2016